 Found 2013 hits with Last Name = 'buchanan' and Initial = 'jl'
Found 2013 hits with Last Name = 'buchanan' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272598
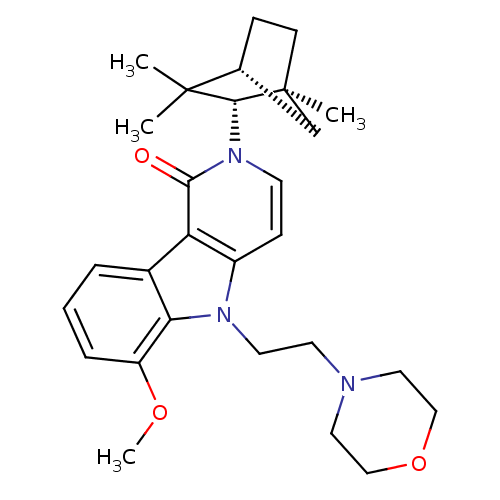
(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272567
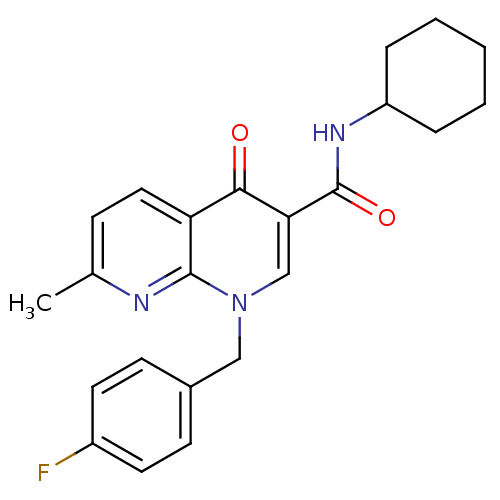
(1-(4-fluorobenzyl)-N-cyclohexyl-7-methyl-4-oxo-1,4...)Show SMILES Cc1ccc2c(n1)n(Cc1ccc(F)cc1)cc(C(=O)NC1CCCCC1)c2=O Show InChI InChI=1S/C23H24FN3O2/c1-15-7-12-19-21(28)20(23(29)26-18-5-3-2-4-6-18)14-27(22(19)25-15)13-16-8-10-17(24)11-9-16/h7-12,14,18H,2-6,13H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50272567

(1-(4-fluorobenzyl)-N-cyclohexyl-7-methyl-4-oxo-1,4...)Show SMILES Cc1ccc2c(n1)n(Cc1ccc(F)cc1)cc(C(=O)NC1CCCCC1)c2=O Show InChI InChI=1S/C23H24FN3O2/c1-15-7-12-19-21(28)20(23(29)26-18-5-3-2-4-6-18)14-27(22(19)25-15)13-16-8-10-17(24)11-9-16/h7-12,14,18H,2-6,13H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50272598

(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50349984
(CHEMBL1813048)Show SMILES CC(=O)Nc1cccc(Nc2ncnc(n2)N2CCC(CC2)OCc2ccc(OC(F)(F)F)cc2)c1C Show InChI InChI=1S/C25H27F3N6O3/c1-16-21(31-17(2)35)4-3-5-22(16)32-23-29-15-30-24(33-23)34-12-10-19(11-13-34)36-14-18-6-8-20(9-7-18)37-25(26,27)28/h3-9,15,19H,10-14H2,1-2H3,(H,31,35)(H,29,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to inactivated human sodium channel Nav1.7 expressed in human HEK293 cells by patch-clamp electrophysiological assay |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379458
(CHEMBL2012273)Show SMILES Fc1c(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)cccc1C(F)(F)F Show InChI InChI=1S/C23H21F4N5OS/c24-20-15(2-1-3-19(20)23(25,26)27)10-32-9-6-18-21(32)28-13-29-22(18)33-17-4-7-31(8-5-17)11-16-12-34-14-30-16/h1-3,6,9,12-14,17H,4-5,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379439
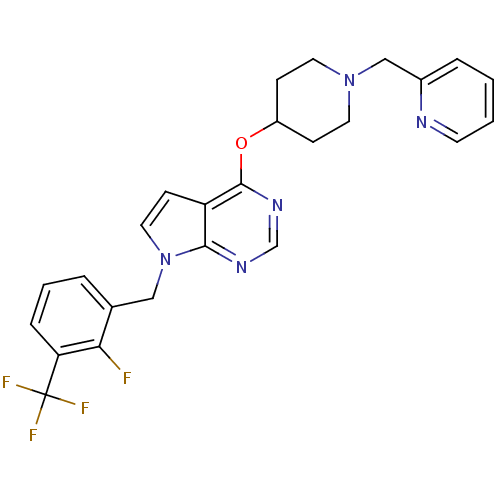
(CHEMBL2012301)Show SMILES Fc1c(Cn2ccc3c(OC4CCN(Cc5ccccn5)CC4)ncnc23)cccc1C(F)(F)F Show InChI InChI=1S/C25H23F4N5O/c26-22-17(4-3-6-21(22)25(27,28)29)14-34-13-9-20-23(34)31-16-32-24(20)35-19-7-11-33(12-8-19)15-18-5-1-2-10-30-18/h1-6,9-10,13,16,19H,7-8,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50349984

(CHEMBL1813048)Show SMILES CC(=O)Nc1cccc(Nc2ncnc(n2)N2CCC(CC2)OCc2ccc(OC(F)(F)F)cc2)c1C Show InChI InChI=1S/C25H27F3N6O3/c1-16-21(31-17(2)35)4-3-5-22(16)32-23-29-15-30-24(33-23)34-12-10-19(11-13-34)36-14-18-6-8-20(9-7-18)37-25(26,27)28/h3-9,15,19H,10-14H2,1-2H3,(H,31,35)(H,29,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-(3-(4-(4-(Benzyloxy)piperidin-1-yl)-1,3,5-triazin-2-ylamino)-phenyl)acetamide from human Nav1.7 expressed in human HEK293 cells... |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50017659
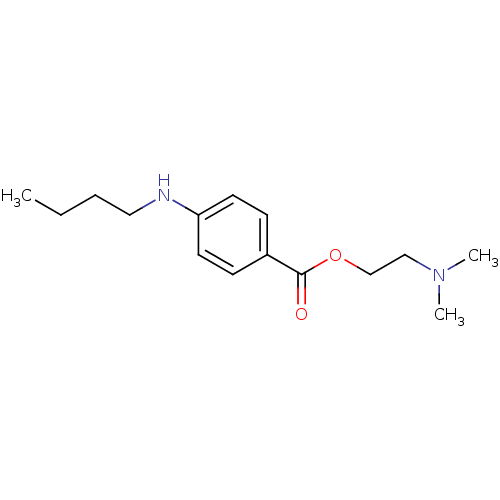
(2-(Dimethylamino)ethyl p-(butylamino)benzoate | 2-...)Show InChI InChI=1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTX from human Nav1.7 expressed in human HEK293 cells after 1 hr by MicroBeta analyzer |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379455
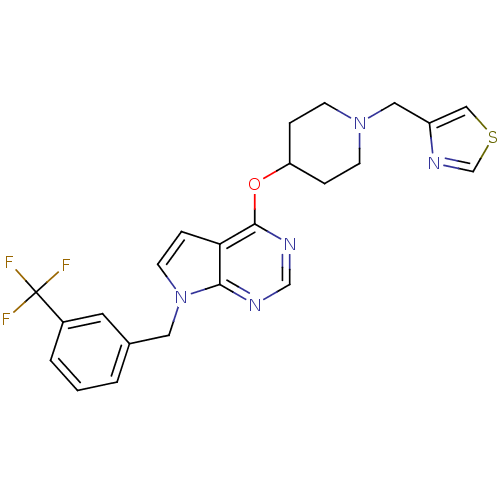
(CHEMBL2012269)Show SMILES FC(F)(F)c1cccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)c1 Show InChI InChI=1S/C23H22F3N5OS/c24-23(25,26)17-3-1-2-16(10-17)11-31-9-6-20-21(31)27-14-28-22(20)32-19-4-7-30(8-5-19)12-18-13-33-15-29-18/h1-3,6,9-10,13-15,19H,4-5,7-8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379438

(CHEMBL2012300)Show SMILES Fc1c(Cl)cccc1Cn1ccc2c(OC3CCN(Cc4ccccn4)CC3)ncnc12 Show InChI InChI=1S/C24H23ClFN5O/c25-21-6-3-4-17(22(21)26)14-31-13-9-20-23(31)28-16-29-24(20)32-19-7-11-30(12-8-19)15-18-5-1-2-10-27-18/h1-6,9-10,13,16,19H,7-8,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379467

(CHEMBL2012293)Show SMILES Cc1cccnc1CN1CCC(CC1)Oc1ncnc2n(Cc3ccccc3)ccc12 Show InChI InChI=1S/C25H27N5O/c1-19-6-5-12-26-23(19)17-29-13-9-21(10-14-29)31-25-22-11-15-30(24(22)27-18-28-25)16-20-7-3-2-4-8-20/h2-8,11-12,15,18,21H,9-10,13-14,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379452
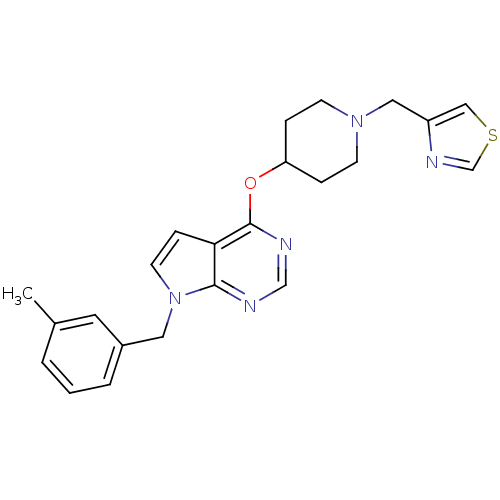
(CHEMBL2012266)Show SMILES Cc1cccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)c1 Show InChI InChI=1S/C23H25N5OS/c1-17-3-2-4-18(11-17)12-28-10-7-21-22(28)24-15-25-23(21)29-20-5-8-27(9-6-20)13-19-14-30-16-26-19/h2-4,7,10-11,14-16,20H,5-6,8-9,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379457
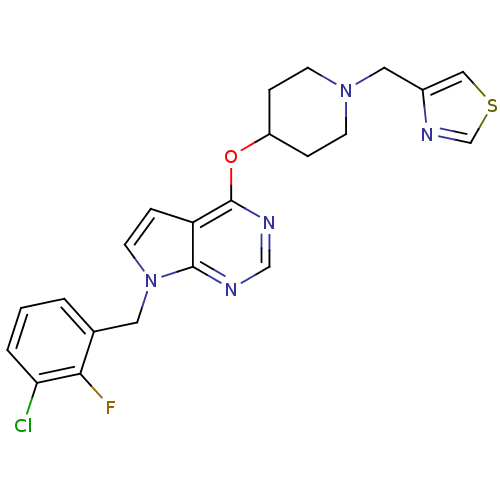
(CHEMBL2012272)Show SMILES Fc1c(Cl)cccc1Cn1ccc2c(OC3CCN(Cc4cscn4)CC3)ncnc12 Show InChI InChI=1S/C22H21ClFN5OS/c23-19-3-1-2-15(20(19)24)10-29-9-6-18-21(29)25-13-26-22(18)30-17-4-7-28(8-5-17)11-16-12-31-14-27-16/h1-3,6,9,12-14,17H,4-5,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379465
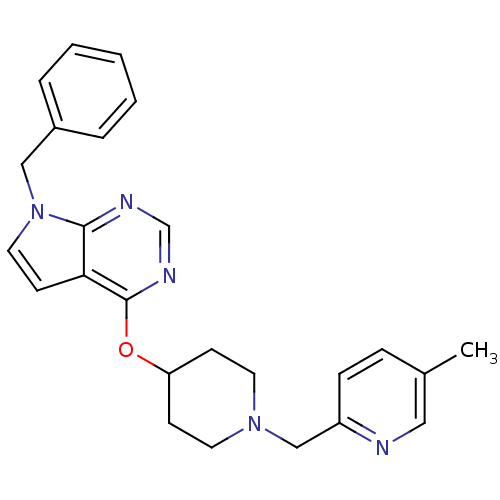
(CHEMBL2012291)Show SMILES Cc1ccc(CN2CCC(CC2)Oc2ncnc3n(Cc4ccccc4)ccc23)nc1 Show InChI InChI=1S/C25H27N5O/c1-19-7-8-21(26-15-19)17-29-12-9-22(10-13-29)31-25-23-11-14-30(24(23)27-18-28-25)16-20-5-3-2-4-6-20/h2-8,11,14-15,18,22H,9-10,12-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379444

(CHEMBL2012246)Show SMILES FC(F)(F)c1nc(OC2CCN(Cc3cscn3)CC2)c2ccn(Cc3ccccc3)c2n1 Show InChI InChI=1S/C23H22F3N5OS/c24-23(25,26)22-28-20-19(8-11-31(20)12-16-4-2-1-3-5-16)21(29-22)32-18-6-9-30(10-7-18)13-17-14-33-15-27-17/h1-5,8,11,14-15,18H,6-7,9-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379437

(CHEMBL2012299)Show SMILES Fc1cccc(Cn2ccc3c(OC4CCN(Cc5ccccn5)CC4)ncnc23)c1F Show InChI InChI=1S/C24H23F2N5O/c25-21-6-3-4-17(22(21)26)14-31-13-9-20-23(31)28-16-29-24(20)32-19-7-11-30(12-8-19)15-18-5-1-2-10-27-18/h1-6,9-10,13,16,19H,7-8,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379471
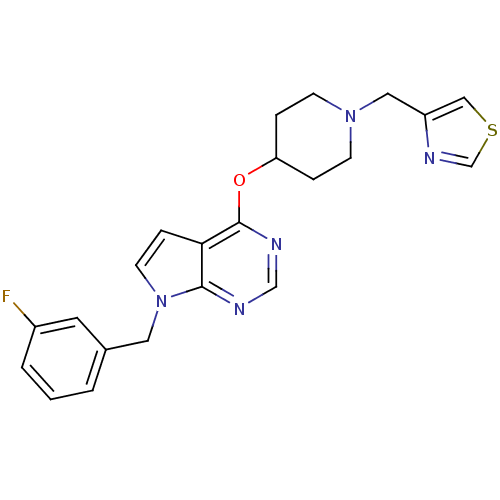
(CHEMBL2012260)Show SMILES Fc1cccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)c1 Show InChI InChI=1S/C22H22FN5OS/c23-17-3-1-2-16(10-17)11-28-9-6-20-21(28)24-14-25-22(20)29-19-4-7-27(8-5-19)12-18-13-30-15-26-18/h1-3,6,9-10,13-15,19H,4-5,7-8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379469
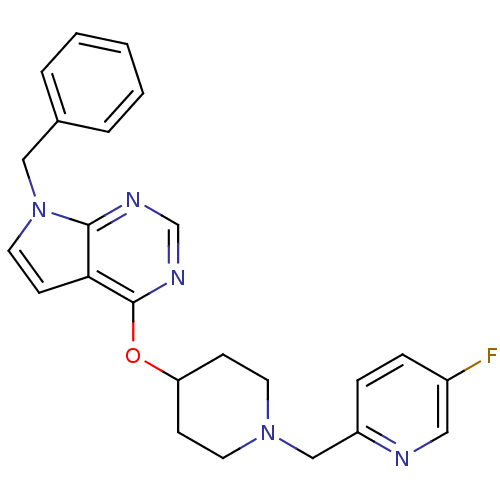
(CHEMBL2012295)Show SMILES Fc1ccc(CN2CCC(CC2)Oc2ncnc3n(Cc4ccccc4)ccc23)nc1 Show InChI InChI=1S/C24H24FN5O/c25-19-6-7-20(26-14-19)16-29-11-8-21(9-12-29)31-24-22-10-13-30(23(22)27-17-28-24)15-18-4-2-1-3-5-18/h1-7,10,13-14,17,21H,8-9,11-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379450

(CHEMBL2012263)Show SMILES Clc1cccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)c1 Show InChI InChI=1S/C22H22ClN5OS/c23-17-3-1-2-16(10-17)11-28-9-6-20-21(28)24-14-25-22(20)29-19-4-7-27(8-5-19)12-18-13-30-15-26-18/h1-3,6,9-10,13-15,19H,4-5,7-8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379470
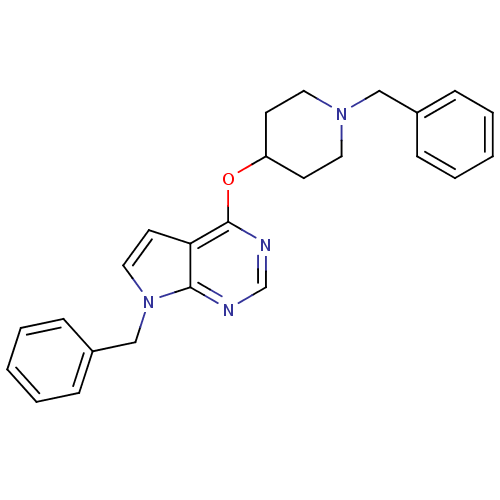
(CHEMBL2012277)Show SMILES C(N1CCC(CC1)Oc1ncnc2n(Cc3ccccc3)ccc12)c1ccccc1 Show InChI InChI=1S/C25H26N4O/c1-3-7-20(8-4-1)17-28-14-11-22(12-15-28)30-25-23-13-16-29(24(23)26-19-27-25)18-21-9-5-2-6-10-21/h1-10,13,16,19,22H,11-12,14-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379440

(CHEMBL2012179)Show SMILES Cc1cn(Cc2ccccc2)c2ncnc(OC3CCN(Cc4cscn4)CC3)c12 Show InChI InChI=1S/C23H25N5OS/c1-17-11-28(12-18-5-3-2-4-6-18)22-21(17)23(25-15-24-22)29-20-7-9-27(10-8-20)13-19-14-30-16-26-19/h2-6,11,14-16,20H,7-10,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379448
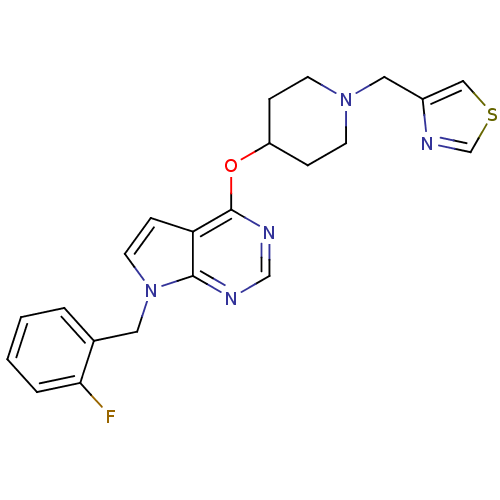
(CHEMBL2012259)Show SMILES Fc1ccccc1Cn1ccc2c(OC3CCN(Cc4cscn4)CC3)ncnc12 Show InChI InChI=1S/C22H22FN5OS/c23-20-4-2-1-3-16(20)11-28-10-7-19-21(28)24-14-25-22(19)29-18-5-8-27(9-6-18)12-17-13-30-15-26-17/h1-4,7,10,13-15,18H,5-6,8-9,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379442

(CHEMBL2012181)Show SMILES Clc1cn(Cc2ccccc2)c2ncnc(OC3CCN(Cc4cscn4)CC3)c12 Show InChI InChI=1S/C22H22ClN5OS/c23-19-12-28(10-16-4-2-1-3-5-16)21-20(19)22(25-14-24-21)29-18-6-8-27(9-7-18)11-17-13-30-15-26-17/h1-5,12-15,18H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379436

(CHEMBL2012298)Show SMILES Fc1ccccc1Cn1ccc2c(OC3CCN(Cc4ccccn4)CC3)ncnc12 Show InChI InChI=1S/C24H24FN5O/c25-22-7-2-1-5-18(22)15-30-14-10-21-23(30)27-17-28-24(21)31-20-8-12-29(13-9-20)16-19-6-3-4-11-26-19/h1-7,10-11,14,17,20H,8-9,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379466
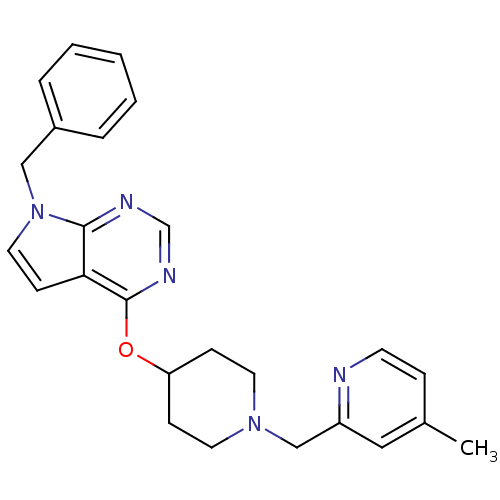
(CHEMBL2012292)Show SMILES Cc1ccnc(CN2CCC(CC2)Oc2ncnc3n(Cc4ccccc4)ccc23)c1 Show InChI InChI=1S/C25H27N5O/c1-19-7-11-26-21(15-19)17-29-12-8-22(9-13-29)31-25-23-10-14-30(24(23)27-18-28-25)16-20-5-3-2-4-6-20/h2-7,10-11,14-15,18,22H,8-9,12-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379456

(CHEMBL2012271)Show SMILES Fc1cccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)c1F Show InChI InChI=1S/C22H21F2N5OS/c23-19-3-1-2-15(20(19)24)10-29-9-6-18-21(29)25-13-26-22(18)30-17-4-7-28(8-5-17)11-16-12-31-14-27-16/h1-3,6,9,12-14,17H,4-5,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379459

(CHEMBL2012274)Show SMILES Fc1cccc(F)c1Cn1ccc2c(OC3CCN(Cc4cscn4)CC3)ncnc12 Show InChI InChI=1S/C22H21F2N5OS/c23-19-2-1-3-20(24)18(19)11-29-9-6-17-21(29)25-13-26-22(17)30-16-4-7-28(8-5-16)10-15-12-31-14-27-15/h1-3,6,9,12-14,16H,4-5,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379464

(CHEMBL2012290)Show SMILES Cc1cccc(CN2CCC(CC2)Oc2ncnc3n(Cc4ccccc4)ccc23)n1 Show InChI InChI=1S/C25H27N5O/c1-19-6-5-9-21(28-19)17-29-13-10-22(11-14-29)31-25-23-12-15-30(24(23)26-18-27-25)16-20-7-3-2-4-8-20/h2-9,12,15,18,22H,10-11,13-14,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379454

(CHEMBL2012268)Show SMILES FC(F)(F)c1ccccc1Cn1ccc2c(OC3CCN(Cc4cscn4)CC3)ncnc12 Show InChI InChI=1S/C23H22F3N5OS/c24-23(25,26)20-4-2-1-3-16(20)11-31-10-7-19-21(31)27-14-28-22(19)32-18-5-8-30(9-6-18)12-17-13-33-15-29-17/h1-4,7,10,13-15,18H,5-6,8-9,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379441

(CHEMBL2012180)Show SMILES Fc1cn(Cc2ccccc2)c2ncnc(OC3CCN(Cc4cscn4)CC3)c12 Show InChI InChI=1S/C22H22FN5OS/c23-19-12-28(10-16-4-2-1-3-5-16)21-20(19)22(25-14-24-21)29-18-6-8-27(9-7-18)11-17-13-30-15-26-17/h1-5,12-15,18H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379431
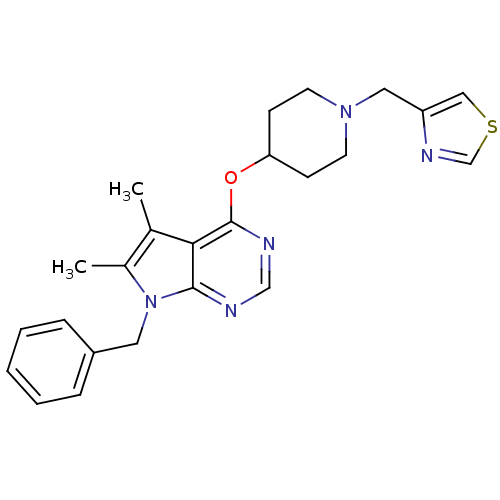
(CHEMBL2012178)Show SMILES Cc1c(C)c2c(OC3CCN(Cc4cscn4)CC3)ncnc2n1Cc1ccccc1 Show InChI InChI=1S/C24H27N5OS/c1-17-18(2)29(12-19-6-4-3-5-7-19)23-22(17)24(26-15-25-23)30-21-8-10-28(11-9-21)13-20-14-31-16-27-20/h3-7,14-16,21H,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379453
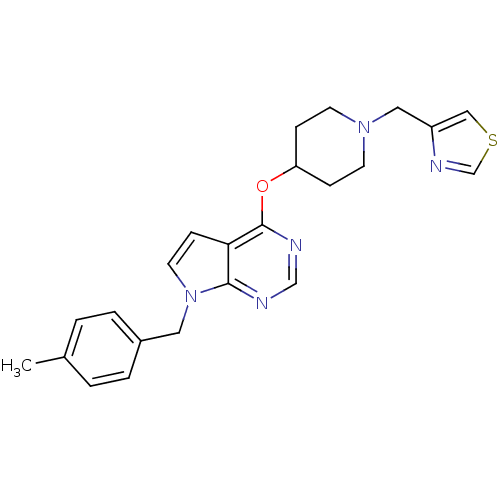
(CHEMBL2012267)Show SMILES Cc1ccc(Cn2ccc3c(OC4CCN(Cc5cscn5)CC4)ncnc23)cc1 Show InChI InChI=1S/C23H25N5OS/c1-17-2-4-18(5-3-17)12-28-11-8-21-22(28)24-15-25-23(21)29-20-6-9-27(10-7-20)13-19-14-30-16-26-19/h2-5,8,11,14-16,20H,6-7,9-10,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379468

(CHEMBL2012294)Show SMILES Fc1cccc(CN2CCC(CC2)Oc2ncnc3n(Cc4ccccc4)ccc23)n1 Show InChI InChI=1S/C24H24FN5O/c25-22-8-4-7-19(28-22)16-29-12-9-20(10-13-29)31-24-21-11-14-30(23(21)26-17-27-24)15-18-5-2-1-3-6-18/h1-8,11,14,17,20H,9-10,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379451
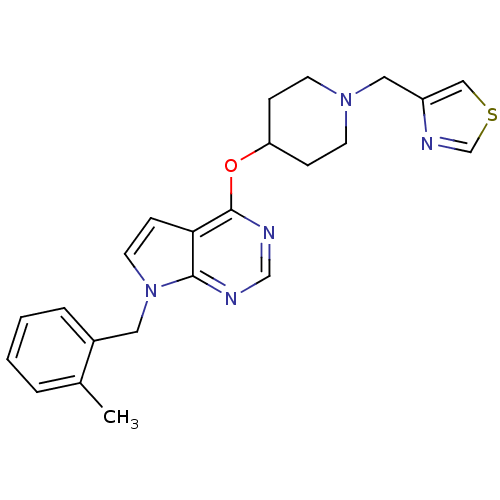
(CHEMBL2012265)Show SMILES Cc1ccccc1Cn1ccc2c(OC3CCN(Cc4cscn4)CC3)ncnc12 Show InChI InChI=1S/C23H25N5OS/c1-17-4-2-3-5-18(17)12-28-11-8-21-22(28)24-15-25-23(21)29-20-6-9-27(10-7-20)13-19-14-30-16-26-19/h2-5,8,11,14-16,20H,6-7,9-10,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 2052-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.015
BindingDB Entry DOI: 10.7270/Q2HM59GC |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50349984

(CHEMBL1813048)Show SMILES CC(=O)Nc1cccc(Nc2ncnc(n2)N2CCC(CC2)OCc2ccc(OC(F)(F)F)cc2)c1C Show InChI InChI=1S/C25H27F3N6O3/c1-16-21(31-17(2)35)4-3-5-22(16)32-23-29-15-30-24(33-23)34-12-10-19(11-13-34)36-14-18-6-8-20(9-7-18)37-25(26,27)28/h3-9,15,19H,10-14H2,1-2H3,(H,31,35)(H,29,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTX from human Nav1.7 expressed in human HEK293 cells after 1 hr by MicroBeta analyzer |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50017659

(2-(Dimethylamino)ethyl p-(butylamino)benzoate | 2-...)Show InChI InChI=1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-(3-(4-(4-(Benzyloxy)piperidin-1-yl)-1,3,5-triazin-2-ylamino)-phenyl)acetamide from human Nav1.7 expressed in human HEK293 cells... |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50117271
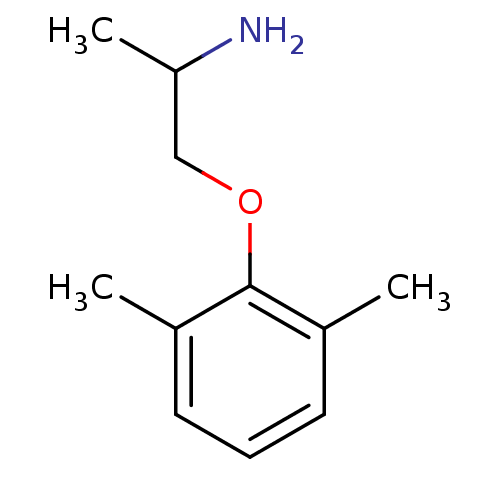
(1-(2,6-dimethylphenoxy)-2-propanolamine | 1-(2,6-d...)Show InChI InChI=1S/C11H17NO/c1-8-5-4-6-9(2)11(8)13-7-10(3)12/h4-6,10H,7,12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to inactivated human sodium channel Nav1.7 expressed in human HEK293 cells by patch-clamp electrophysiological assay |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362558
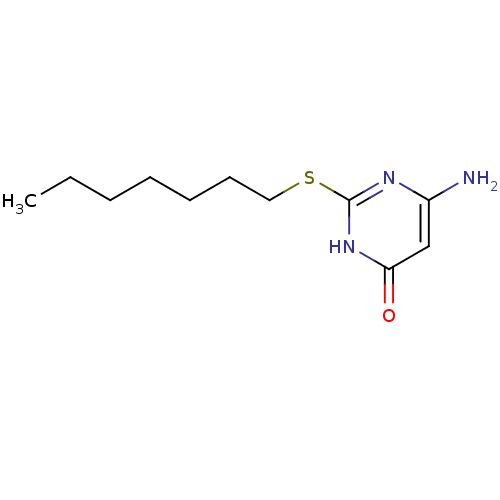
(CHEMBL1425238)Show InChI InChI=1S/C11H19N3OS/c1-2-3-4-5-6-7-16-11-13-9(12)8-10(15)14-11/h8H,2-7H2,1H3,(H3,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 1055-60 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.111
BindingDB Entry DOI: 10.7270/Q2CV4J7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362580
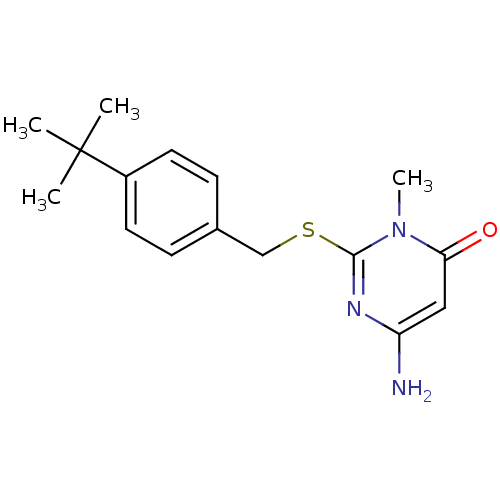
(CHEMBL1938867)Show InChI InChI=1S/C16H21N3OS/c1-16(2,3)12-7-5-11(6-8-12)10-21-15-18-13(17)9-14(20)19(15)4/h5-9H,10,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 1055-60 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.111
BindingDB Entry DOI: 10.7270/Q2CV4J7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362567

(CHEMBL1313991)Show InChI InChI=1S/C15H19N3OS/c1-15(2,3)11-6-4-10(5-7-11)9-20-14-17-12(16)8-13(19)18-14/h4-8H,9H2,1-3H3,(H3,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from human ERG |
Bioorg Med Chem Lett 22: 1055-60 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.111
BindingDB Entry DOI: 10.7270/Q2CV4J7X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50194684
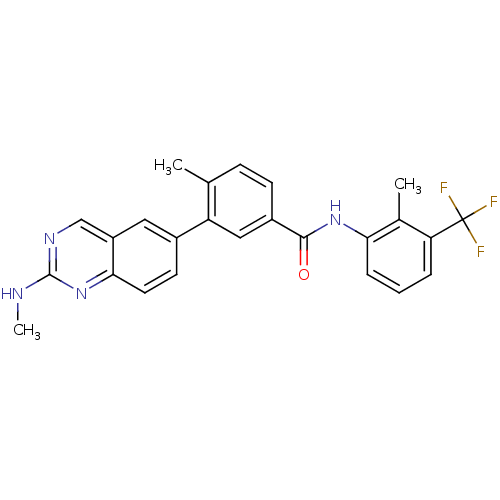
(4-methyl-N-(2-methyl-3-4-methyl-N-(2-methyl-3-(2-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C25H21F3N4O/c1-14-7-8-17(23(33)31-21-6-4-5-20(15(21)2)25(26,27)28)12-19(14)16-9-10-22-18(11-16)13-30-24(29-3)32-22/h4-13H,1-3H3,(H,31,33)(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck by HTRF kinase assay |
J Med Chem 49: 5671-86 (2006)
Article DOI: 10.1021/jm0605482
BindingDB Entry DOI: 10.7270/Q29G5MFD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325]
(Homo sapiens (Human)) | BDBM259592

(US9505749, 104)Show SMILES O=C(CCSc1nc2ccccc2c(=O)[nH]1)N[C@H]1CC[C@@H](CC1)n1c2ccc(cc2[nH]c1=O)C#N |r,wU:17.18,wD:20.25,(-2.42,-1.15,;-2.42,-2.69,;-3.75,-3.47,;-5.09,-2.69,;-5.09,-1.15,;-6.42,-.38,;-7.75,-1.15,;-9.09,-.38,;-10.42,-1.15,;-11.75,-.38,;-11.75,1.15,;-10.42,1.93,;-9.09,1.15,;-7.75,1.93,;-7.75,3.47,;-6.42,1.15,;-1.08,-3.47,;.25,-2.69,;.25,-1.15,;1.58,-.38,;2.92,-1.15,;2.92,-2.69,;1.58,-3.47,;4.25,-.38,;5.72,-.86,;6.34,-2.27,;7.87,-2.43,;8.78,-1.18,;8.15,.22,;6.62,.38,;5.72,1.63,;4.25,1.15,;2.92,1.93,;10.27,-1.58,;11.75,-1.98,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... |
US Patent US9505749 (2016)
BindingDB Entry DOI: 10.7270/Q2FN154F |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325]
(Homo sapiens (Human)) | BDBM259576

(US9505749, 87)Show SMILES Clc1ccc2n([C@H]3CC[C@@H](CC3)NC(=O)CSc3nc4ccccc4c(=O)[nH]3)c(=O)[nH]c2c1 |r,wU:6.5,wD:9.12,(-.35,-6.38,;.74,-5.29,;.26,-3.82,;1.29,-2.68,;2.8,-3,;4.04,-2.09,;4.04,-.55,;2.71,.22,;2.71,1.76,;4.04,2.53,;5.38,1.76,;5.38,.22,;4.04,4.07,;2.71,4.84,;2.71,6.38,;1.38,4.07,;.04,4.84,;-1.29,4.07,;-2.62,4.84,;-3.96,4.07,;-5.29,4.84,;-6.62,4.07,;-6.62,2.53,;-5.29,1.76,;-3.96,2.53,;-2.62,1.76,;-2.62,.22,;-1.29,2.53,;5.29,-3,;6.62,-2.23,;4.81,-4.46,;3.27,-4.46,;2.24,-5.61,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0597 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... |
US Patent US9505749 (2016)
BindingDB Entry DOI: 10.7270/Q2FN154F |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325]
(Homo sapiens (Human)) | BDBM259607
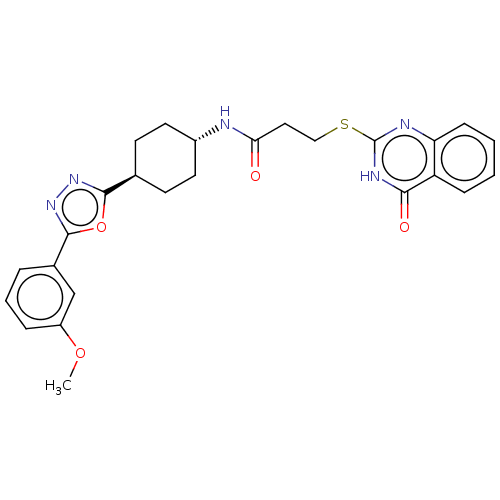
(US9505749, 120)Show SMILES COc1cccc(c1)-c1nnc(o1)[C@H]1CC[C@@H](CC1)NC(=O)CCSc1nc2ccccc2c(=O)[nH]1 |r,wU:16.21,wD:13.14,(11.82,-3.63,;10.33,-3.23,;9.93,-1.74,;11.02,-.65,;10.62,.84,;9.13,1.24,;8.04,.15,;8.44,-1.34,;6.56,.55,;5.65,1.79,;4.19,1.32,;4.19,-.22,;5.65,-.7,;2.85,-.99,;1.52,-.22,;.19,-.99,;.19,-2.53,;1.52,-3.3,;2.85,-2.53,;-1.15,-3.3,;-2.48,-2.53,;-2.48,-.99,;-3.81,-3.3,;-5.15,-2.53,;-5.15,-.99,;-6.48,-.22,;-7.82,-.99,;-9.15,-.22,;-10.48,-.99,;-11.82,-.22,;-11.82,1.32,;-10.48,2.09,;-9.15,1.32,;-7.82,2.09,;-7.82,3.63,;-6.48,1.32,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0647 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... |
US Patent US9505749 (2016)
BindingDB Entry DOI: 10.7270/Q2FN154F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50194688
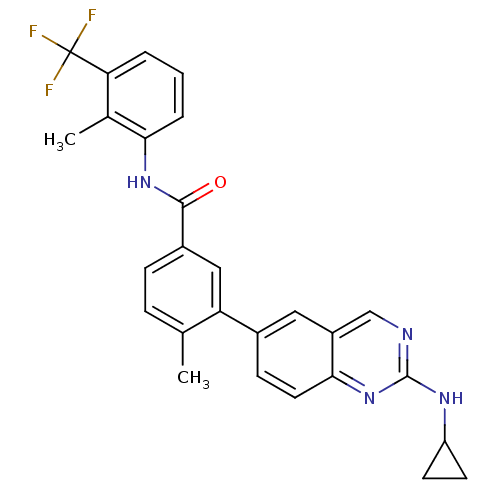
(4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...)Show SMILES Cc1ccc(cc1-c1ccc2nc(NC3CC3)ncc2c1)C(=O)Nc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C27H23F3N4O/c1-15-6-7-18(25(35)33-23-5-3-4-22(16(23)2)27(28,29)30)13-21(15)17-8-11-24-19(12-17)14-31-26(34-24)32-20-9-10-20/h3-8,11-14,20H,9-10H2,1-2H3,(H,33,35)(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lck by HTRF kinase assay |
J Med Chem 49: 5671-86 (2006)
Article DOI: 10.1021/jm0605482
BindingDB Entry DOI: 10.7270/Q29G5MFD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325]
(Homo sapiens (Human)) | BDBM259608

(US9505749, 121)Show SMILES COc1ccc(cc1)-c1nnc(o1)[C@H]1CC[C@@H](CC1)NC(=O)CCSc1nc2ccccc2c(=O)[nH]1 |r,wU:16.21,wD:13.14,(12.71,-.12,;11.62,-1.21,;10.13,-.81,;9.04,-1.9,;7.55,-1.5,;7.15,-.01,;8.24,1.08,;9.73,.68,;5.67,.38,;4.76,1.63,;3.3,1.15,;3.3,-.38,;4.76,-.86,;1.96,-1.15,;.63,-.38,;-.7,-1.15,;-.7,-2.69,;.63,-3.47,;1.96,-2.69,;-2.04,-3.47,;-3.37,-2.69,;-3.37,-1.15,;-4.7,-3.47,;-6.04,-2.69,;-6.04,-1.15,;-7.37,-.38,;-8.71,-1.15,;-10.04,-.38,;-11.37,-1.15,;-12.71,-.38,;-12.71,1.15,;-11.37,1.93,;-10.04,1.15,;-8.71,1.93,;-8.71,3.47,;-7.37,1.15,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0724 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... |
US Patent US9505749 (2016)
BindingDB Entry DOI: 10.7270/Q2FN154F |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2 [946-1162]
(Homo sapiens (Human)) | BDBM259594

(US9505749, 107)Show SMILES O=C(CCSc1nc2ccccc2c(=O)[nH]1)N[C@H]1CC[C@@H](CC1)Oc1ccccn1 |r,wU:17.18,wD:20.25,(2.67,5,;1.33,4.23,;,5,;-1.33,4.23,;-2.67,5,;-4,4.23,;-4,2.69,;-5.33,1.93,;-5.33,.38,;-6.67,-.38,;-8,.38,;-8,1.93,;-6.67,2.69,;-6.67,4.23,;-8,5,;-5.33,5,;1.33,2.69,;2.67,1.93,;4,2.69,;5.33,1.93,;5.33,.38,;4,-.38,;2.67,.38,;6.67,-.38,;6.67,-1.93,;8,-2.69,;8,-4.23,;6.67,-5,;5.33,-4.23,;5.33,-2.69,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0882 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... |
US Patent US9505749 (2016)
BindingDB Entry DOI: 10.7270/Q2FN154F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data