

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Histone-lysine N-methyltransferase, H3 lysine-79 specific | ||
| Ligand | BDBM297390 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Assays of Enzymatic Activity | ||
| IC50 | 0.730±n/a nM | ||
| Citation |  Olhava, EJ; Chesworth, R; Pollock, RM; Jin, L Inhibitors of protein methyltransferase DOT1L and methods of use thereof US Patent US10112968 Publication Date 10/30/2018 Olhava, EJ; Chesworth, R; Pollock, RM; Jin, L Inhibitors of protein methyltransferase DOT1L and methods of use thereof US Patent US10112968 Publication Date 10/30/2018 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific | |||
| Name: | Histone-lysine N-methyltransferase, H3 lysine-79 specific | ||
| Synonyms: | 2.1.1.43 | DOT1-like protein | DOT1-like protein (Dot1L) | DOT1L | DOT1L_HUMAN | H3-K79-HMTase | Histone H3-K79 methyltransferase | Histone H3-K79 methyltransferase (DOT1L) | Histone Methyltransferase DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific (DOT1L) | KIAA1814 | KMT4 | Lysine N-methyltransferase 4 | ||
| Type: | Protein | ||
| Mol. Mass.: | 184911.91 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q8TEK3 | ||
| Residue: | 1537 | ||
| Sequence: |
| ||
| BDBM297390 | |||
| n/a | |||
| Name | BDBM297390 | ||
| Synonyms: | (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1s,3R)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol | US10112968, Compound A3 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H42N8O3 | ||
| Mol. Mass. | 562.7063 | ||
| SMILES | CC(C)N(CC1OC([C@H](O)C1O)n1cnc2c(N)ncnc12)C1CC(CCc2nc3ccc(cc3[nH]2)C(C)(C)C)C1 |r,wU:8.8,(-11.05,-1.3,;-9.51,-1.3,;-8.74,.03,;-8.74,-2.64,;-9.51,-3.97,;-11.05,-3.97,;-11.96,-2.72,;-13.42,-3.2,;-13.42,-4.74,;-14.67,-5.65,;-11.96,-5.22,;-11.48,-6.68,;-14.67,-2.3,;-14.67,-.76,;-16.13,-.28,;-17.04,-1.53,;-18.57,-1.69,;-19.47,-.44,;-19.19,-3.09,;-18.29,-4.34,;-16.76,-4.18,;-16.13,-2.77,;-7.2,-2.64,;-6.11,-1.55,;-5.02,-2.64,;-3.48,-2.64,;-2.71,-1.3,;-1.17,-1.3,;-.27,-2.55,;1.2,-2.07,;2.53,-2.84,;3.86,-2.07,;3.86,-.53,;2.53,.24,;1.2,-.53,;-.27,-.06,;5.2,.24,;6.53,1.01,;5.97,-1.1,;4.43,1.57,;-6.11,-3.73,)| | ||
| Structure | 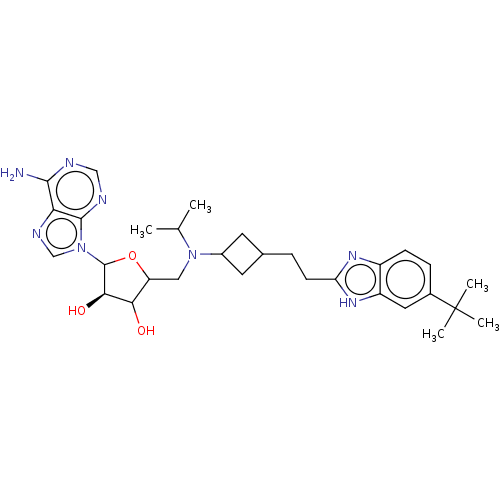
| ||