

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92649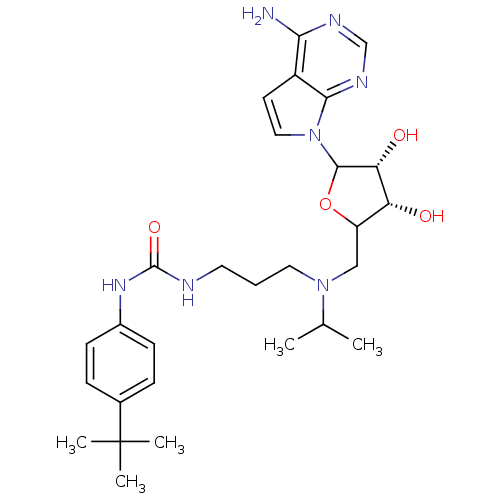 (EPZ004777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297395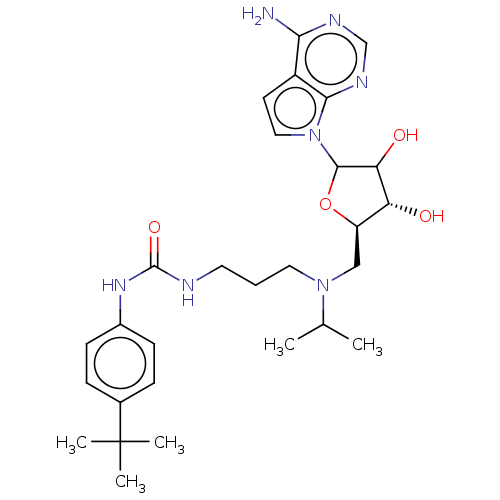 (US10112968, Compound D16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | 0.0100 | n/a | 0.000300 | 3.00E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92648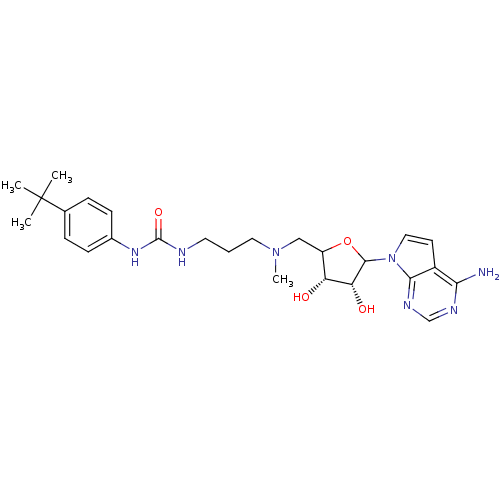 (EPZ004450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92647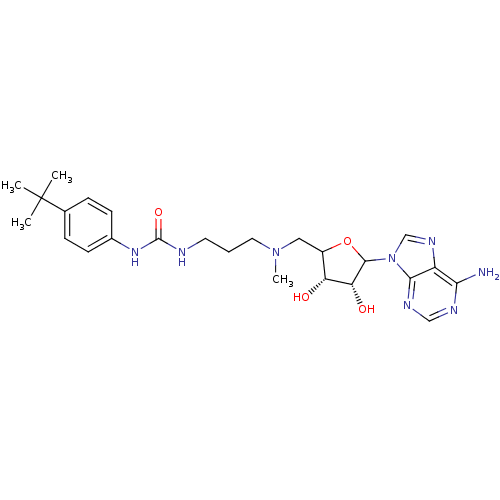 (EPZ003696) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297394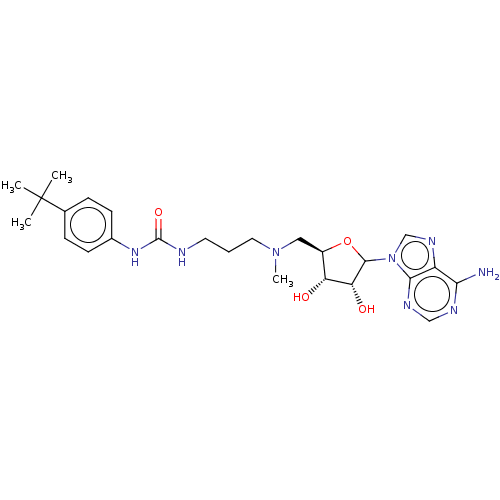 (US10112968, Compound C118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | 1.70 | n/a | 0.0200 | 1.20E+7 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92642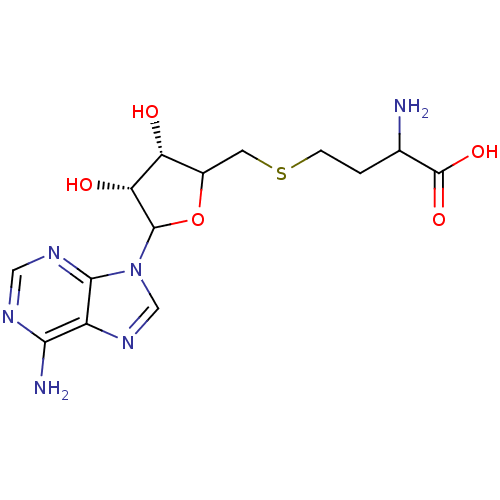 (SAH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297392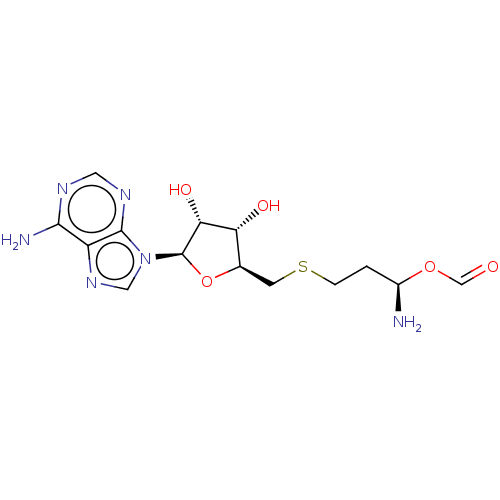 (US10112968, Compound SAH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 320 | n/a | n/a | 71 | n/a | 0.100 | 1.40E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297393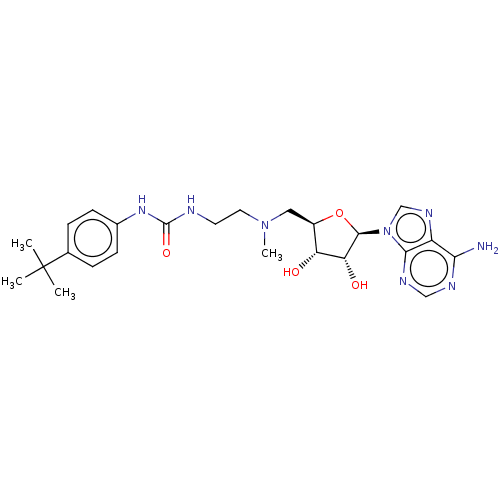 (US10112968, Compound C94) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 845 | n/a | n/a | 167 | n/a | 0.200 | 1.20E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92646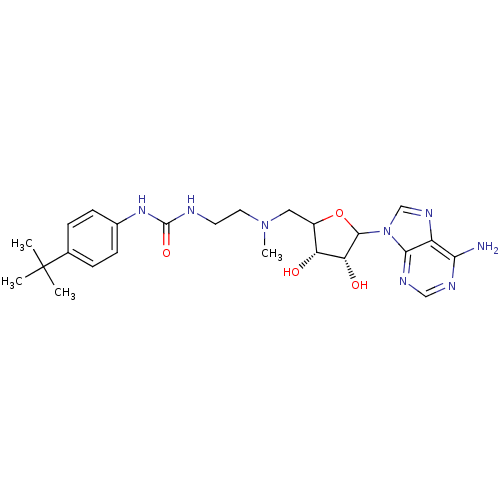 (EPZ003647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 845 | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92644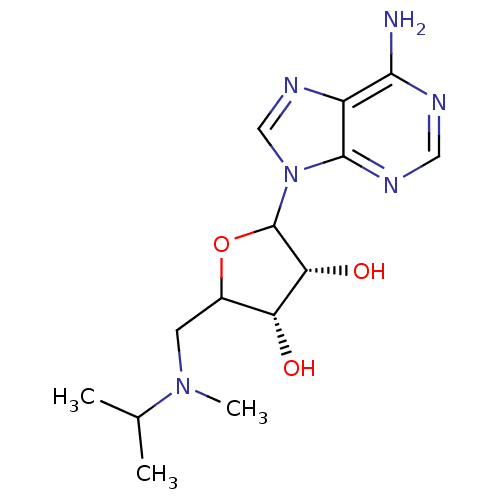 (EPZ002446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92645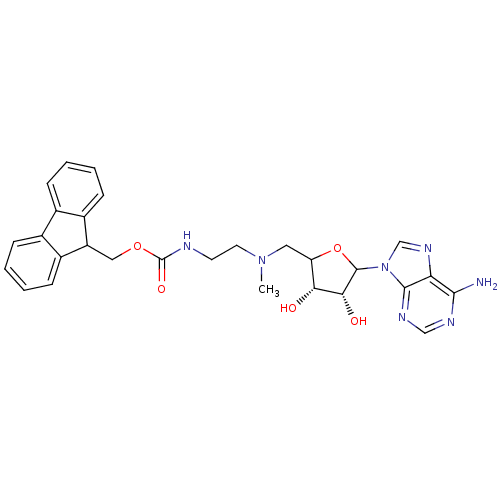 (EPZ003144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92643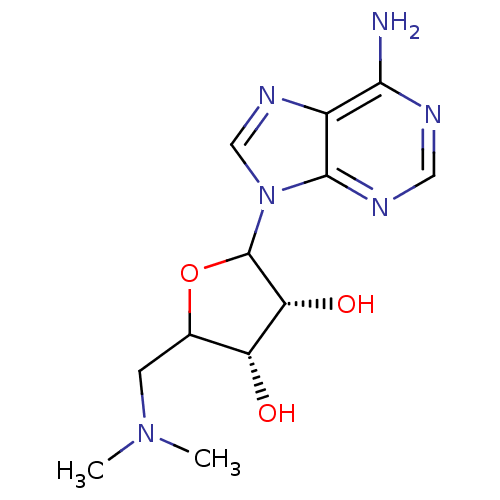 (EPZ000004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244794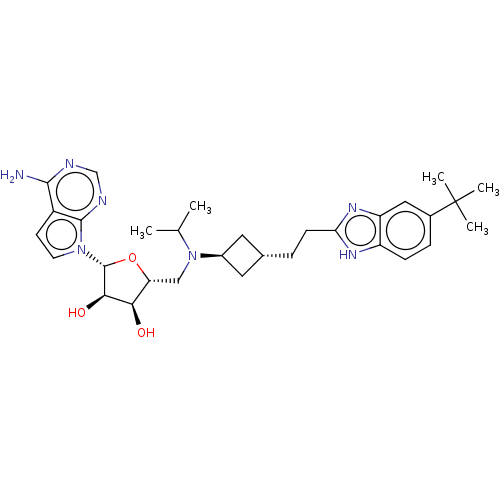 (US9446064, A75) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244792 (US9446064, A5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297391 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244795 (US9446064, A86) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244791 (US9446064, A3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297390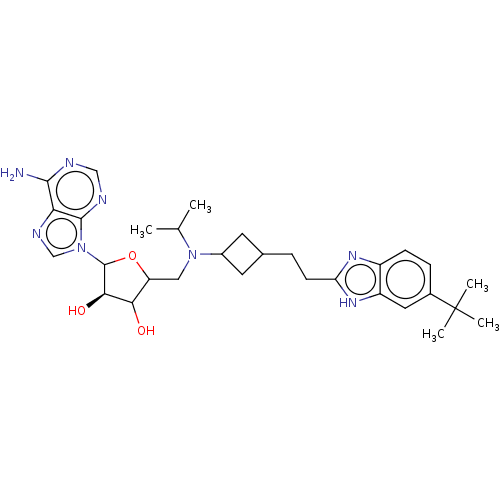 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1s,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3 fold in DMSO for 10 points and 1 μl was plated in a 384 well microtiter plate. Positive control (100% inh... | US Patent US10143704 (2018) BindingDB Entry DOI: 10.7270/Q2FB551H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297389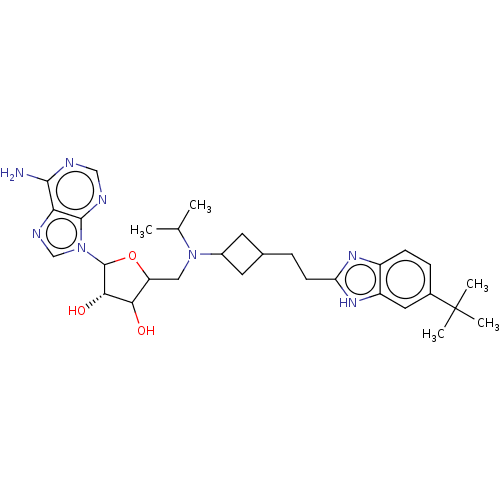 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244796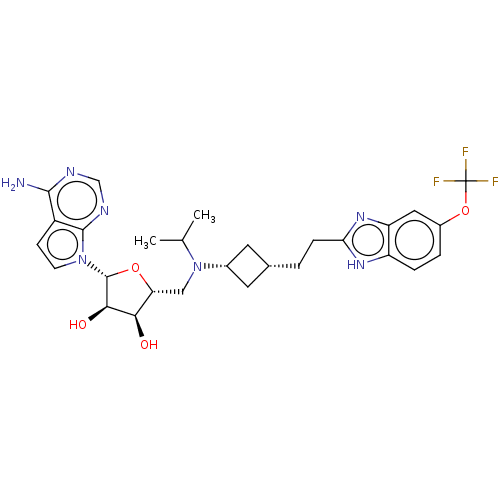 (US9446064, A87) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244797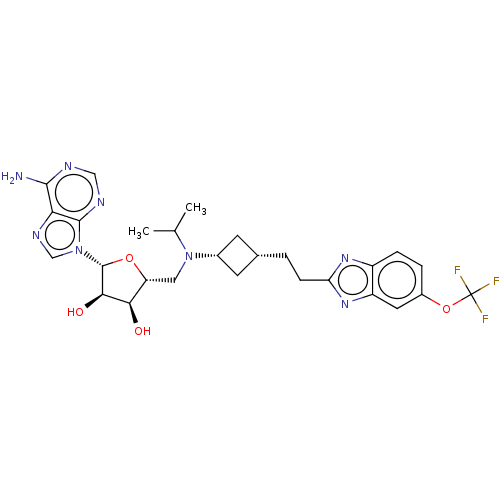 (US9446064, A91) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244793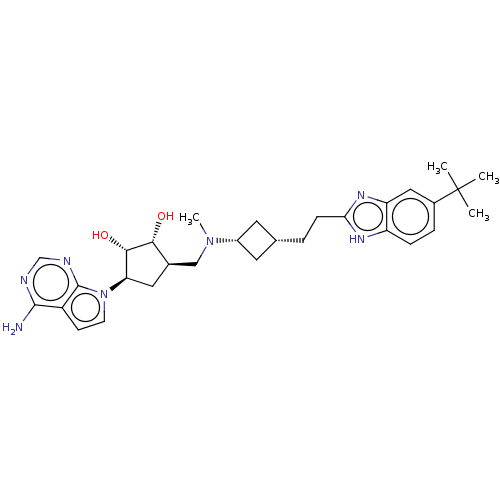 (US9446064, A69) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244798 (US9446064, A93) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.92 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM178085 (US10307413, Compound 188 | US10391089, Compound 18...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM178085 (US10307413, Compound 188 | US10391089, Compound 18...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM177922 (US10307413, Compound 337 | US10391089, Compound 16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149902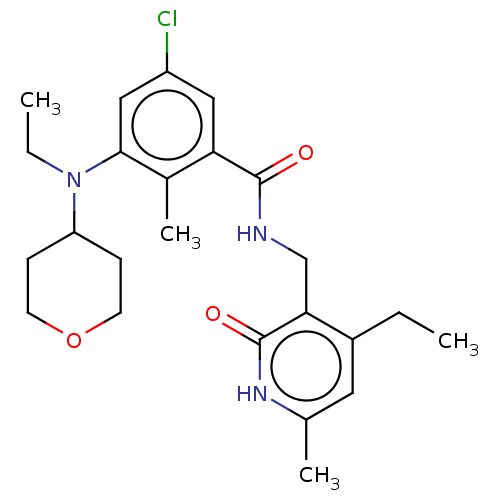 (CHEMBL3771372) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149895 (CHEMBL3770000) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149889 (CHEMBL3769571) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM336999 (US9745291, Compound 269 | US9745291, Compound 45 |...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176872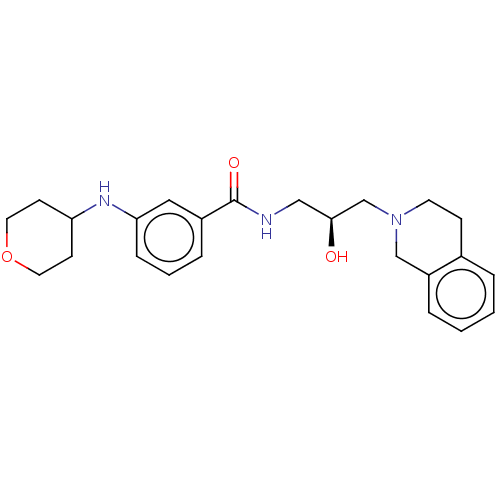 (US10307413, Compound 44 | US10391089, Compound 44 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176841 (US10307413, Compound 20 | US10391089, Compound 20 ...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176815 (US10307413, Compound 1 | US10391089, Compound 1 | ...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM336999 (US9745291, Compound 269 | US9745291, Compound 45 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176872 (US10307413, Compound 44 | US10391089, Compound 44 ...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM190198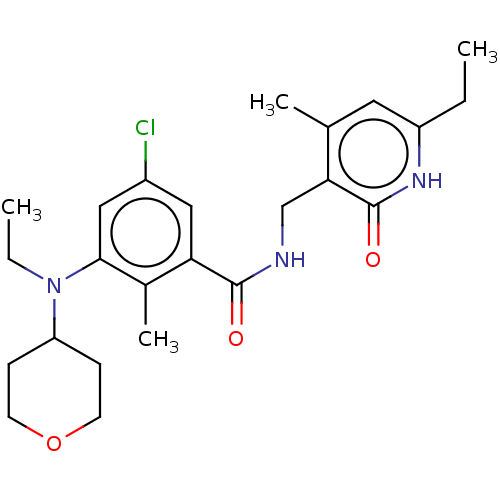 (EPZ008279 | US9175331, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM177922 (US10307413, Compound 337 | US10391089, Compound 16...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM336960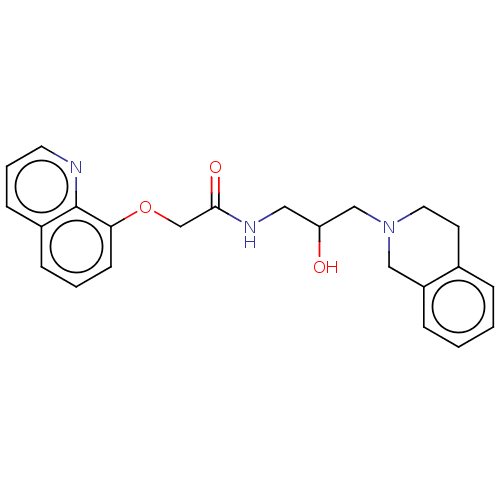 (US9745291, Compound 6 | US9765068, Compound 6) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149884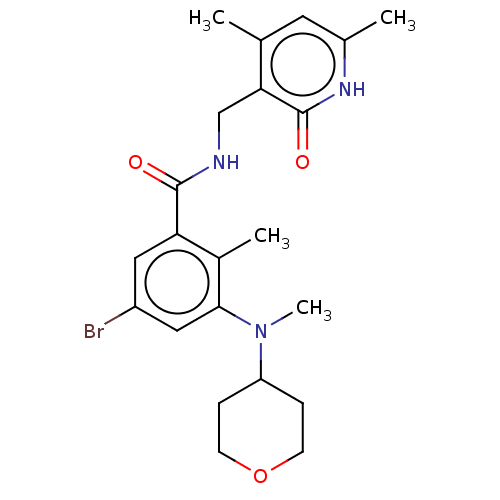 (CHEMBL3770973) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149882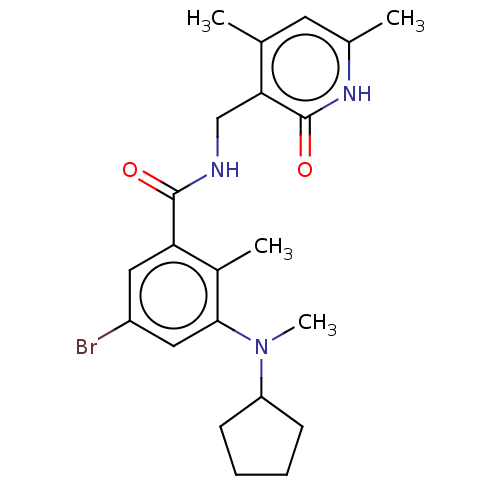 (CHEMBL3771083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149901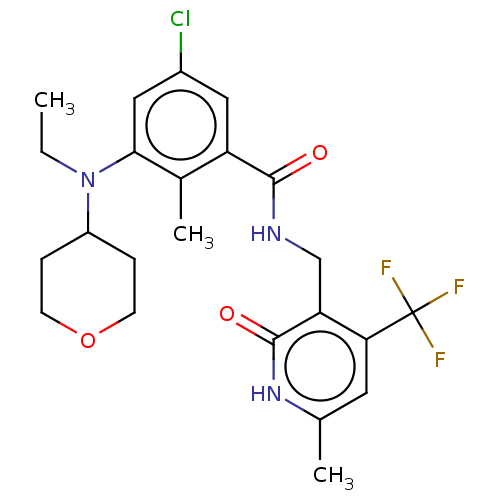 (CHEMBL3770820) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149896 (CHEMBL3771013) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176841 (US10307413, Compound 20 | US10391089, Compound 20 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM343824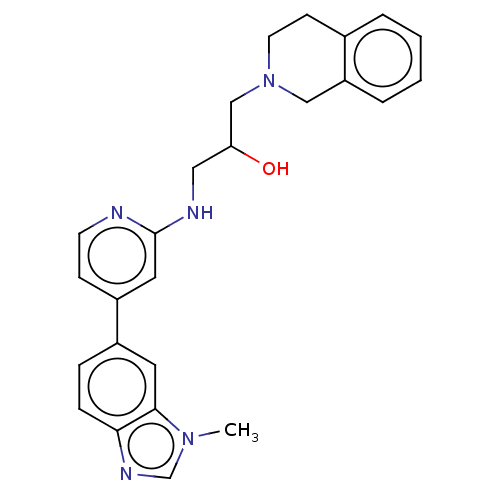 (1-(3,4-Dihydroisoquinolin-2(1H)-yl)-3-((4-(1-methy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length FLAG-tagged PRMT5/human His6-tagged MEP50 expressed in baculovirus-infected Sf9 cells assessed as reduction in tritiu... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM336960 (US9745291, Compound 6 | US9765068, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM176815 (US10307413, Compound 1 | US10391089, Compound 1 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT5 in human Z138 cells assessed as reduction in symmetrical dimethylation of arginine containing substrate using SmD3 as substrate i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00380 BindingDB Entry DOI: 10.7270/Q2SQ941H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149706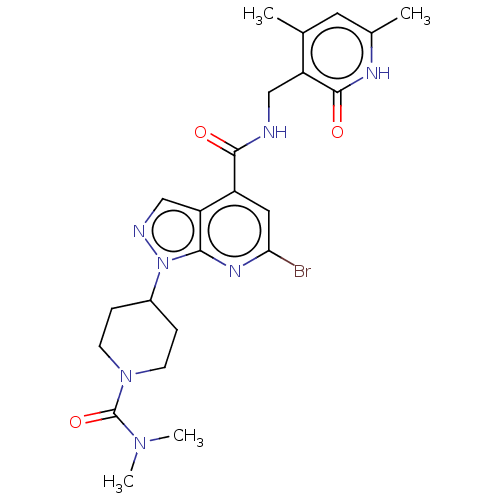 (CHEMBL3769652) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50149705 (CHEMBL3771343) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay | J Med Chem 59: 1556-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01501 BindingDB Entry DOI: 10.7270/Q2B56MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 129 total ) | Next | Last >> |