

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297395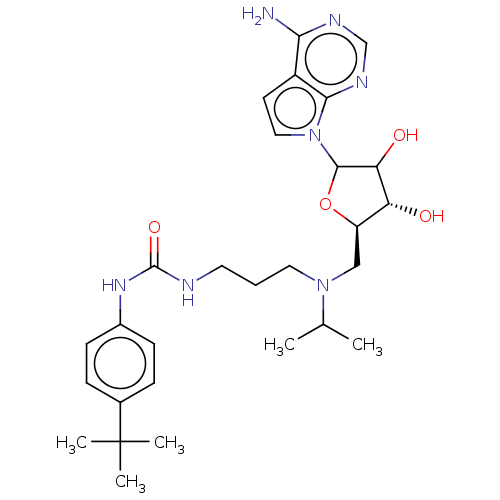 (US10112968, Compound D16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | 0.0100 | n/a | 0.000300 | 3.00E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92649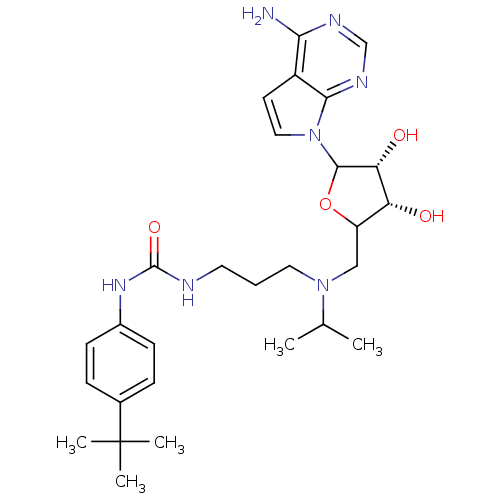 (EPZ004777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462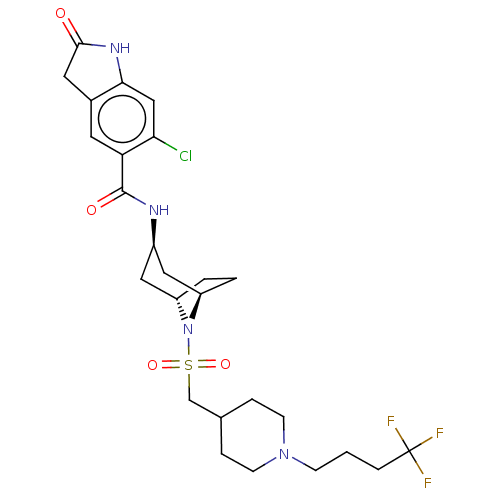 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92648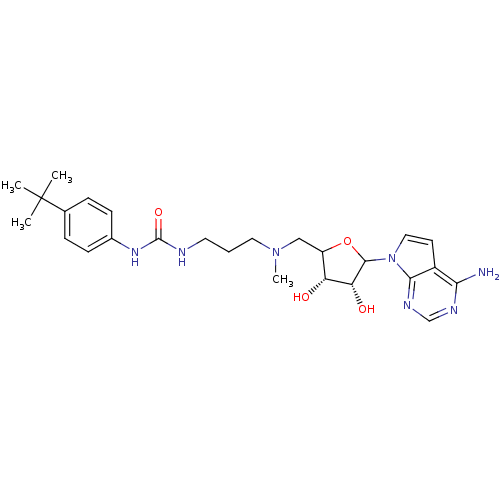 (EPZ004450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Mixed type inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed N-ter... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297394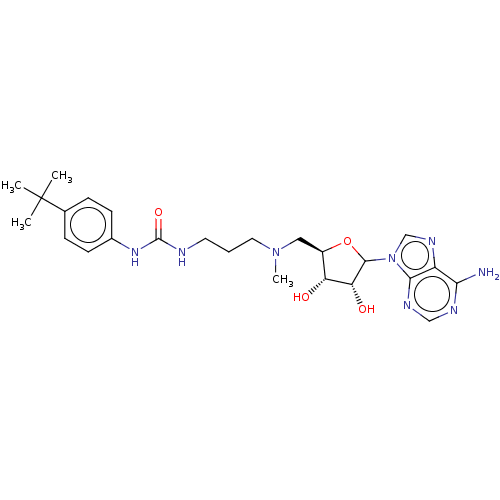 (US10112968, Compound C118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | 1.70 | n/a | 0.0200 | 1.20E+7 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92647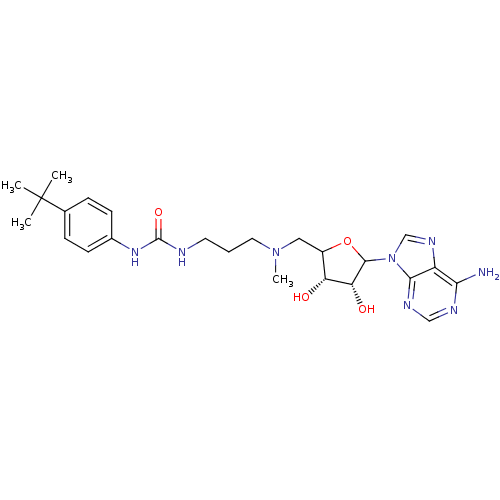 (EPZ003696) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297392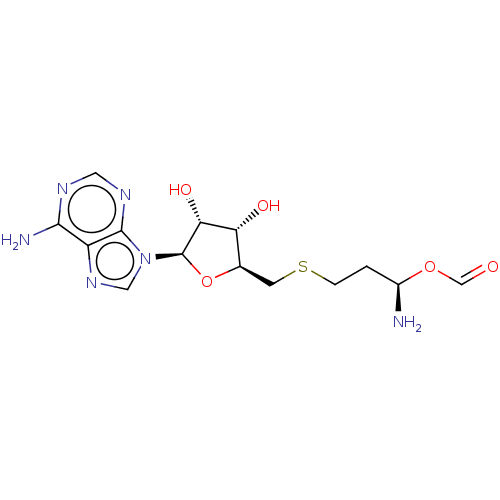 (US10112968, Compound SAH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 320 | n/a | n/a | 71 | n/a | 0.100 | 1.40E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92642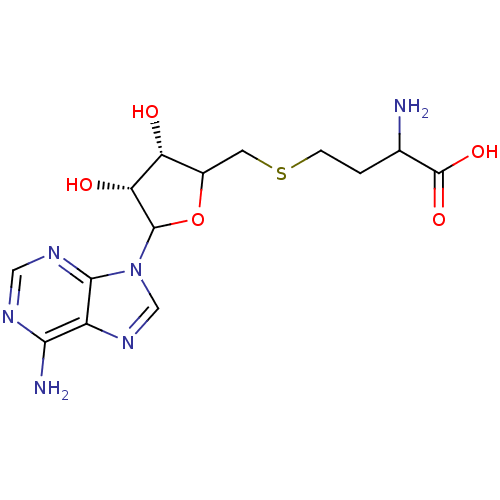 (SAH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92646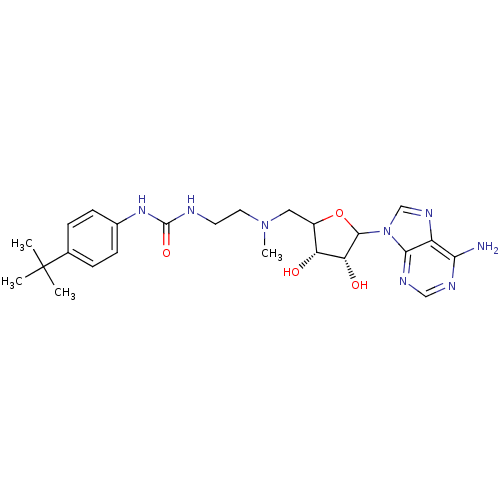 (EPZ003647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 845 | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297393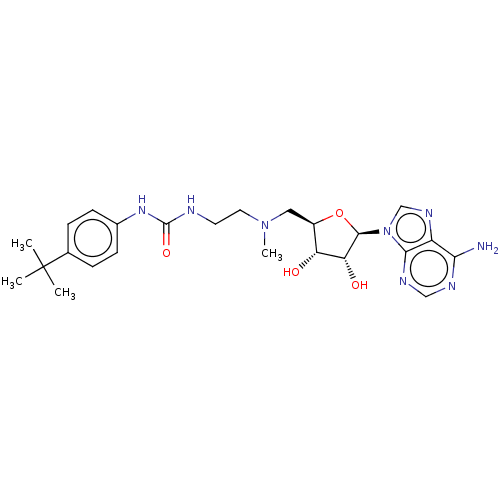 (US10112968, Compound C94) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 845 | n/a | n/a | 167 | n/a | 0.200 | 1.20E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92644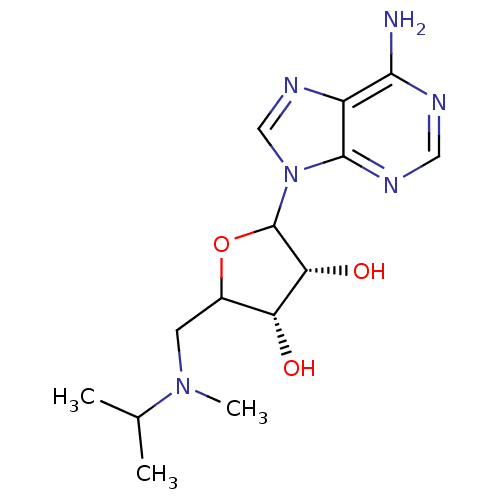 (EPZ002446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92645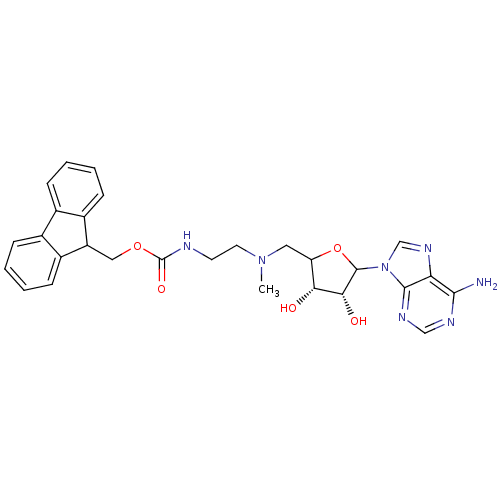 (EPZ003144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92643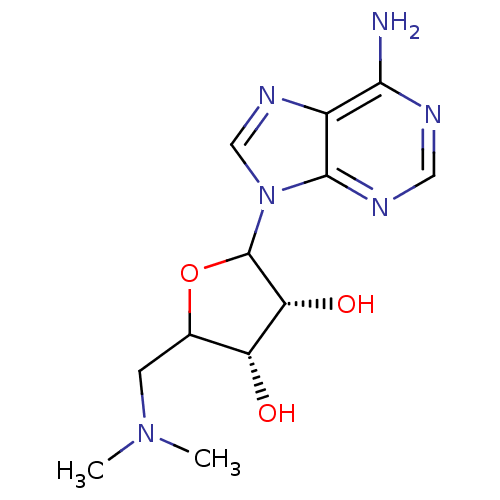 (EPZ000004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433063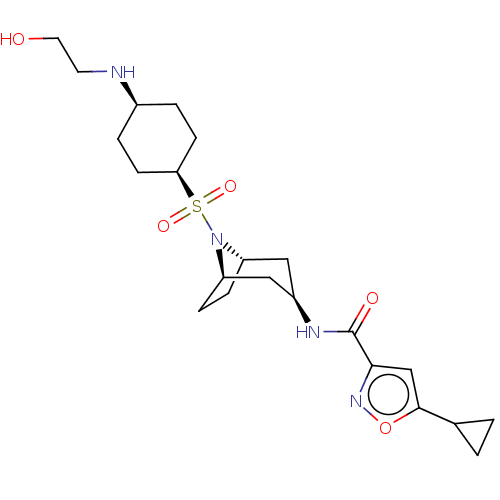 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1S,4S)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085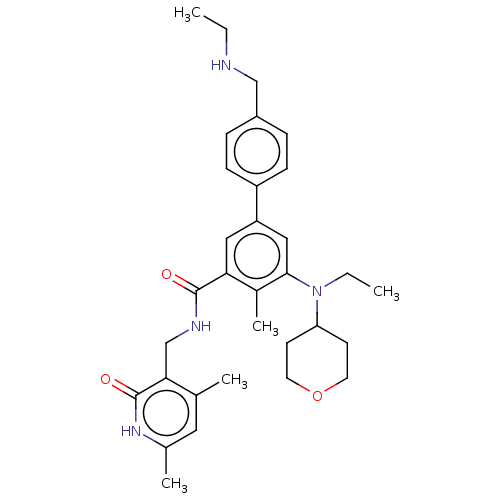 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172085 (US10155002, Compound 91 | US11052093, Compound 91 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297391 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432825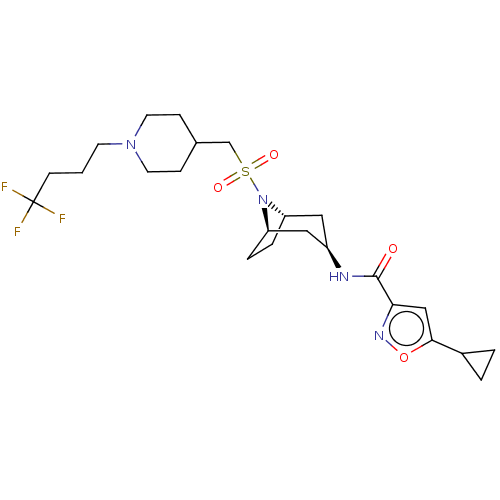 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(4,4,4- trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433064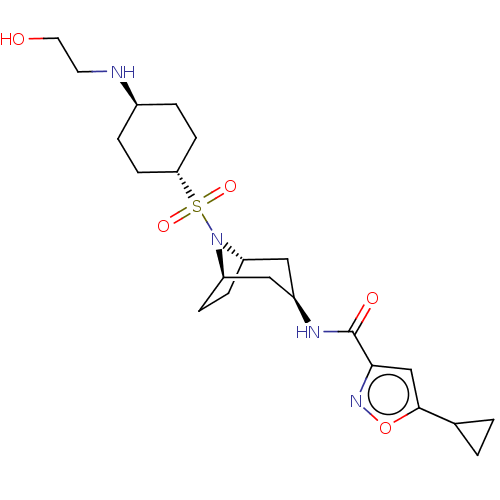 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1R,4R)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297390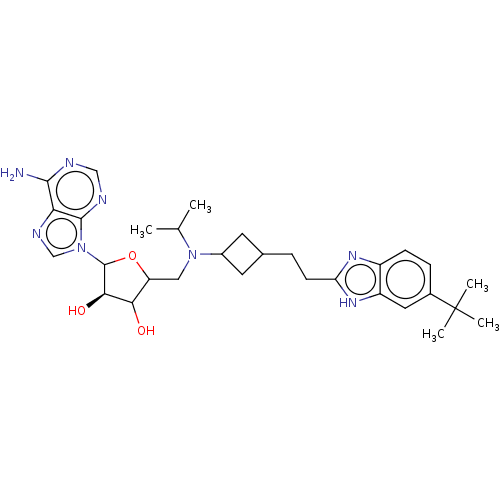 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1s,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297389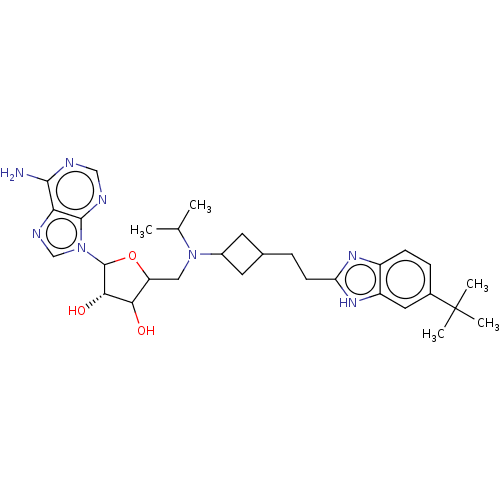 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]17beta-estradiol from recombinant human ERalpha expressed in 293T cells | Bioorg Med Chem Lett 15: 5562-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.010 BindingDB Entry DOI: 10.7270/Q2N879CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163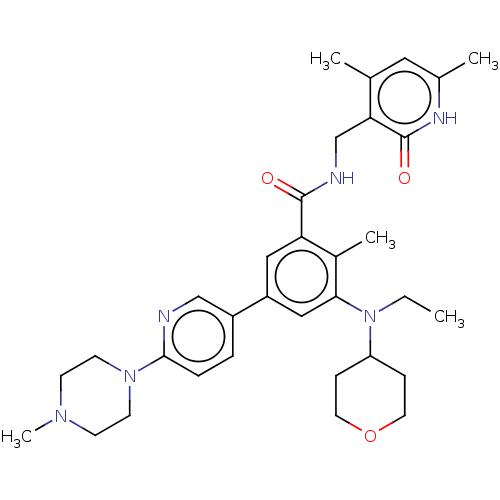 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433065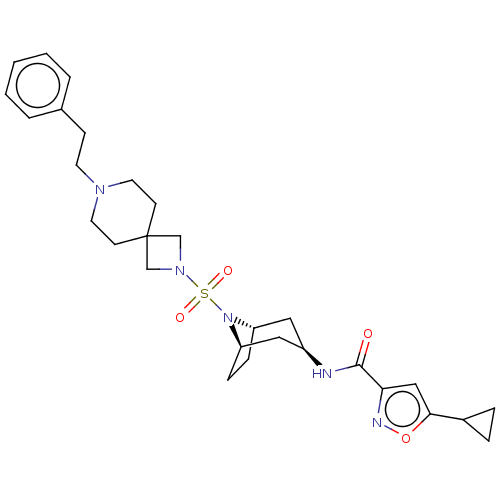 (5-cyclopropyl-N-((1R,3r,5S)-8-((7- phenethyl-2,7-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172163 (US10155002, Compound 171 | US11052093, Compound 17...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432826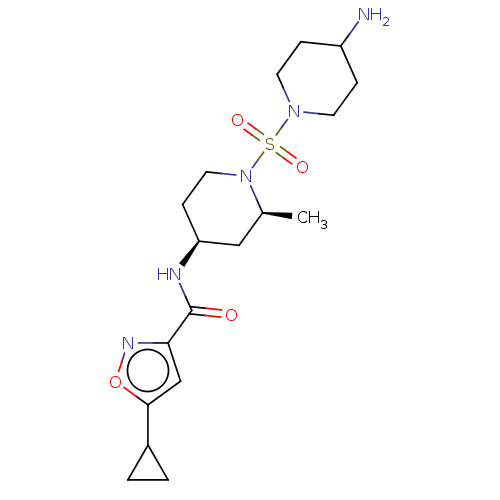 (N-((2S,4S)-1-((4-aminopiperidin-1- yl)sulfonyl)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432822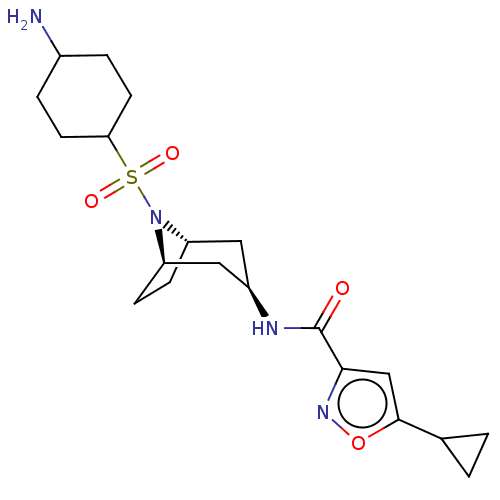 (N-((1R,3r,5S)-8-((4- aminocyclohexyl)sulfonyl)-8- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087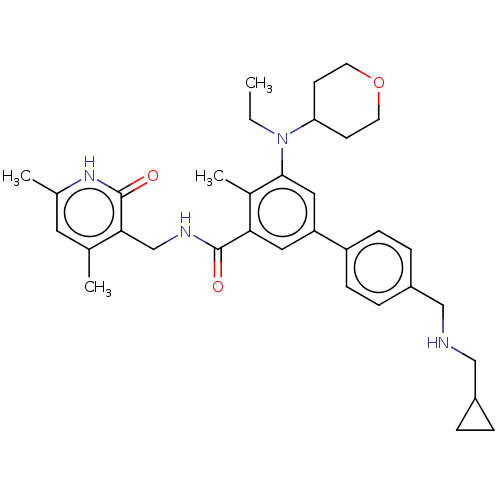 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172087 (US10155002, Compound 93 | US11052093, Compound 93 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433066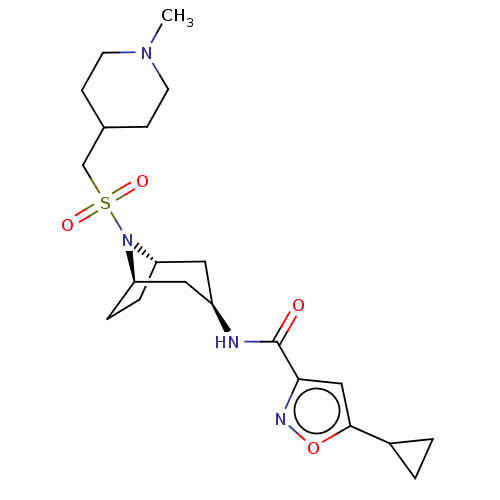 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1- methylpiperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432829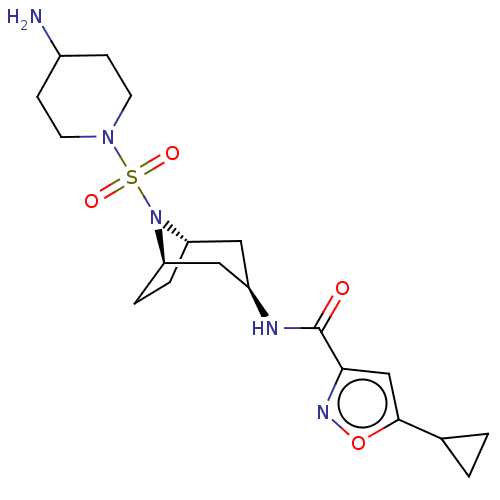 (N-((1R,3r,5S)-8-((4-aminopiperidin-1- yl)sulfonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432828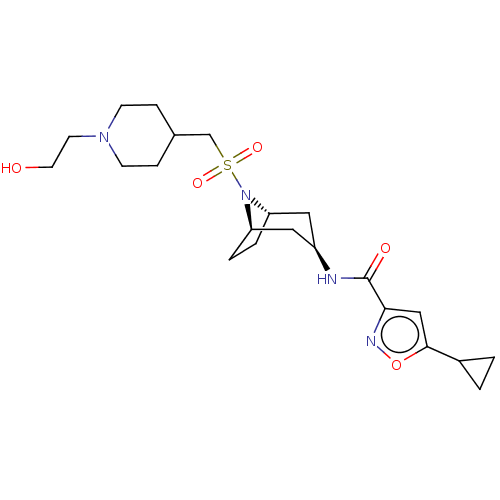 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(2- hydroxyethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432827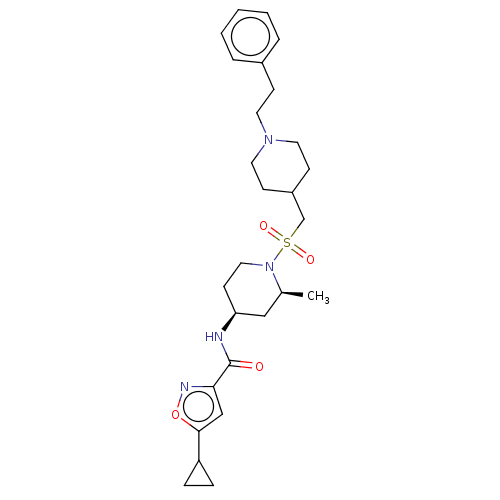 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-phenethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176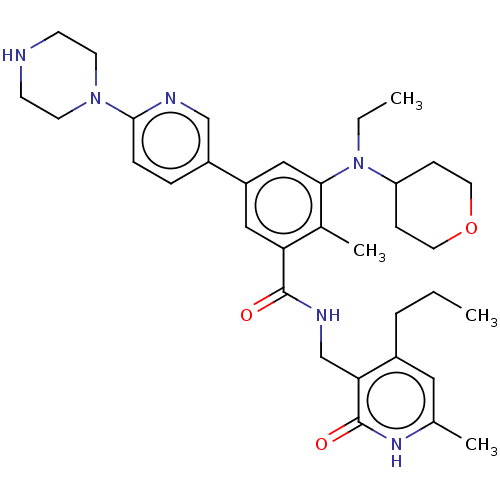 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172176 (EPZ009161 | US10155002, Compound 184 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433067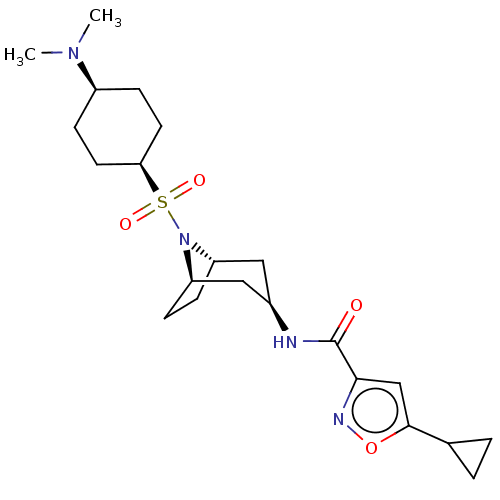 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1s,4S)- 4-(dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433068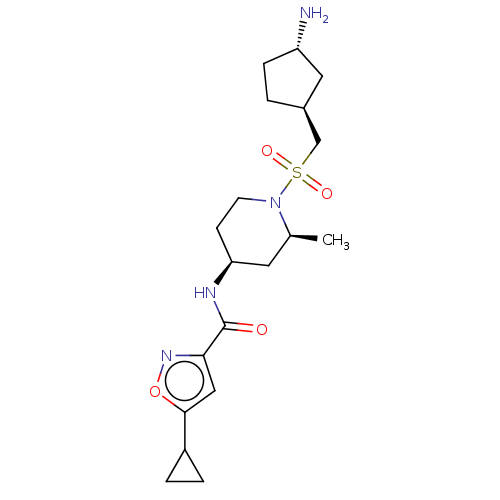 (N-((2S,4S)-1-((((1S,3S)-3- aminocyclopentyl)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446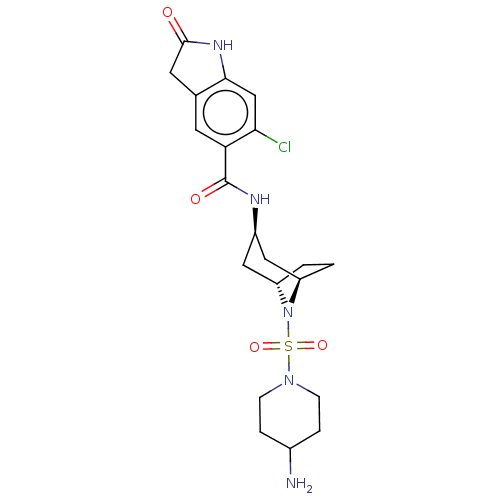 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432830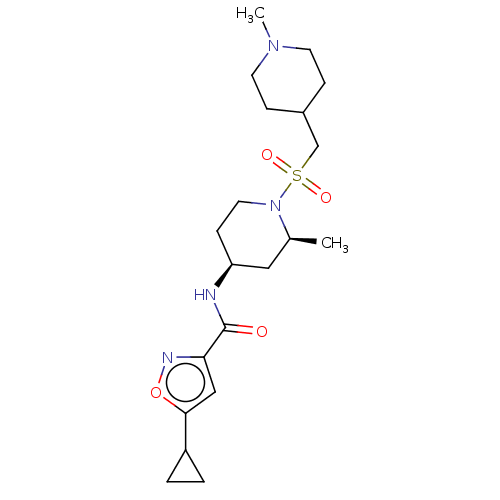 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-methylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432823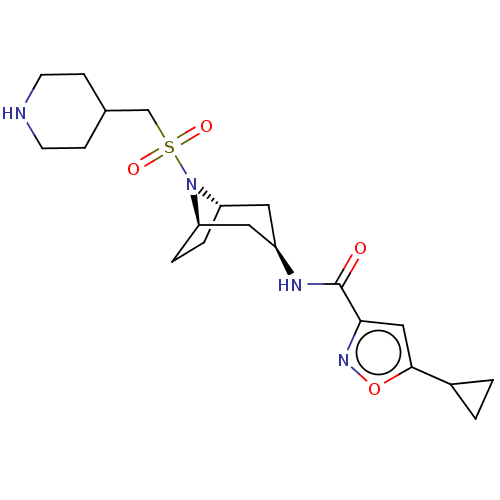 (5-cyclopropyl-N-((1R,3r,5S)-8- ((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444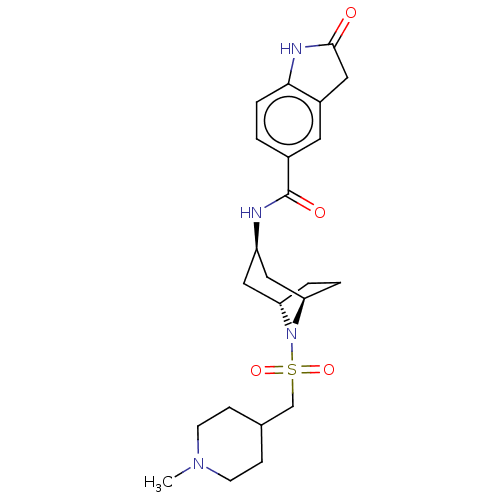 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7770 total ) | Next | Last >> |