

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | ||
| Ligand | BDBM445086 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | UCHL1 Biochemical IC50 Assay | ||
| IC50 | 550±n/a nM | ||
| Citation |  Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020 Kemp, M; Stockley, M; Jones, A Cyanopyrrolidines as dub inhibitors for the treatment of cancer US Patent US10669234 Publication Date 6/2/2020 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Ubiquitin carboxyl-terminal hydrolase isozyme L1 | |||
| Name: | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | ||
| Synonyms: | Neuron cytoplasmic protein 9.5 | PGP 9.5 | PGP9.5 | UCH-L1 | UCHL1 | UCHL1_HUMAN | Ubiquitin thioesterase L1 | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 24819.03 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | ChEMBL_974327 | ||
| Residue: | 223 | ||
| Sequence: |
| ||
| BDBM445086 | |||
| n/a | |||
| Name | BDBM445086 | ||
| Synonyms: | (S)-2-(4-(3,5-dimethyl- 1H-pyrazol-4-yl)indoline- 1-carbonyl)pyrrolidine- 1-carbonitrile | US10669234, Example 25 | US11319287, Example 25 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C19H21N5O | ||
| Mol. Mass. | 335.4029 | ||
| SMILES | Cc1n[nH]c(C)c1-c1cccc2N(CCc12)C(=O)[C@@H]1CCCN1C#N |wU:18.20,(4.04,-2.74,;2.58,-3.22,;2.1,-4.68,;.56,-4.68,;.09,-3.22,;-1.38,-2.74,;1.33,-2.31,;1.33,-.77,;2.67,,;2.67,1.54,;1.33,2.31,;,1.54,;-1.46,2.02,;-2.37,.77,;-1.46,-.48,;;-1.94,3.48,;-3.45,3.8,;-.91,4.63,;.62,4.46,;1.25,5.87,;.1,6.9,;-1.23,6.13,;-2.64,6.76,;-4.04,7.38,)| | ||
| Structure | 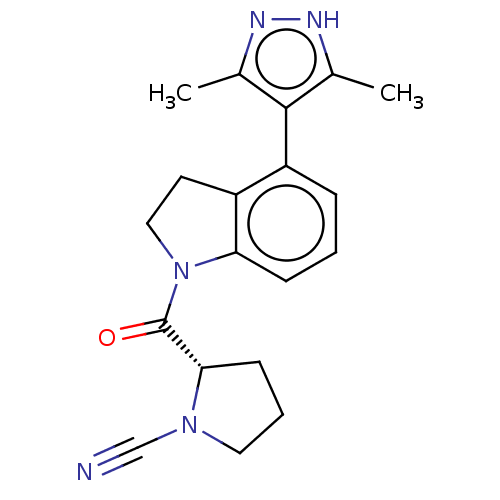
| ||