null
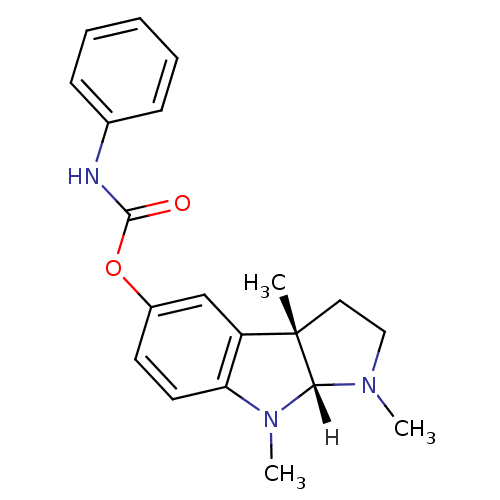
SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C
InChI Key InChIKey=PBHFNBQPZCRWQP-QUCCMNQESA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 10958
Found 19 hits for monomerid = 10958
Affinity DataIC50: 24nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibitory activity against Butyrylcholinesterase in plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibitory activity against Acetylcholinesterase in cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibitory activity against Acetylcholinesterase in electric eelMore data for this Ligand-Target Pair
Affinity DataIC50: 11.9nMAssay Description:Inhibition of rat brain AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 104nMAssay Description:Inhibition of rat plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 22.1nMAssay Description:Inhibition of human AChE by Ellmans testMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibitory concentration against human plasma ButyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibitory activity against Acetylcholinesterase in cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity against Acetylcholinesterase in cortexMore data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibition of BuChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair