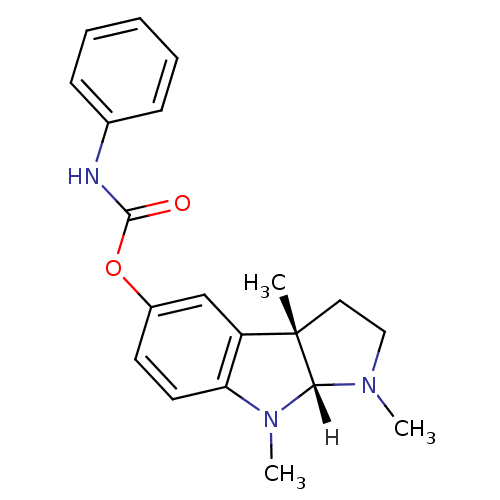
Affinity DataIC50: 11.9nMAssay Description:Inhibition of rat brain AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibitory activity against Acetylcholinesterase in cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against human erythrocyte AcetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 22.1nMAssay Description:Inhibition of human AChE by Ellmans testMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibitory activity against Acetylcholinesterase in cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibitory activity against Acetylcholinesterase in electric eelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair