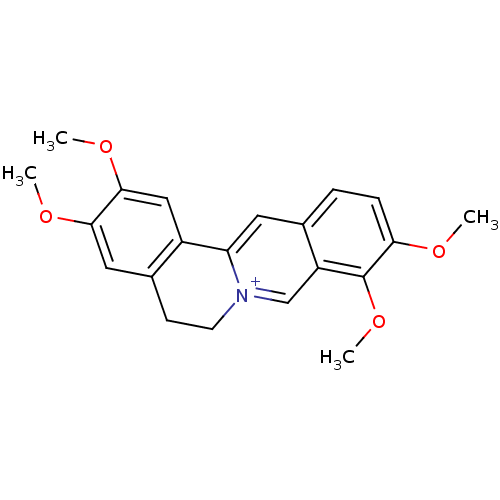
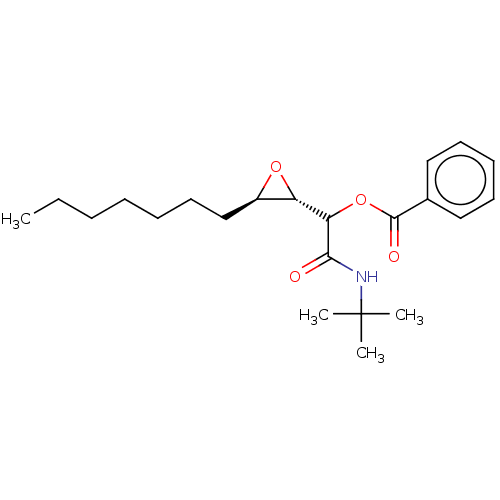
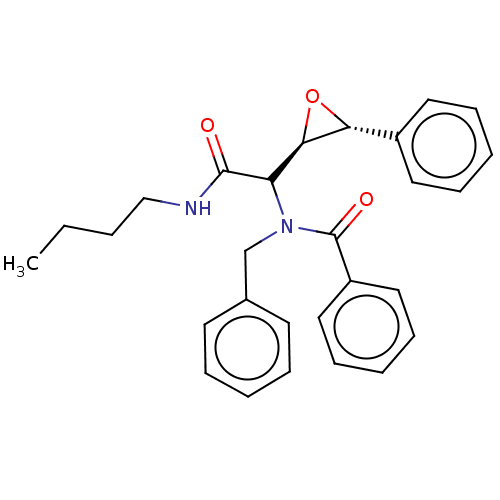
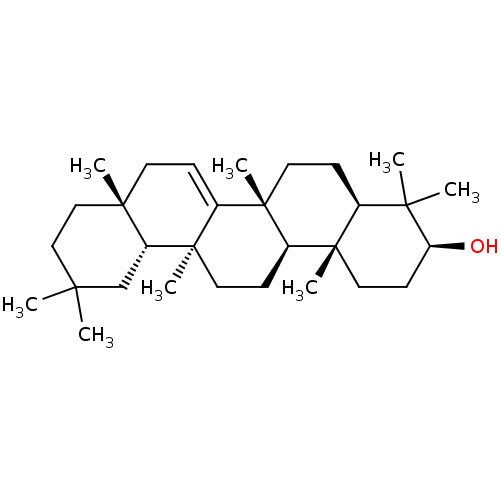
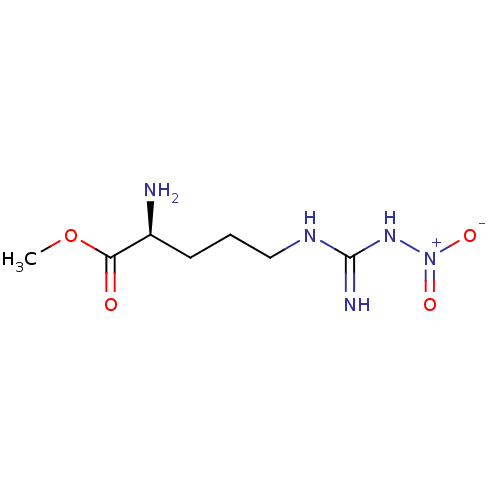
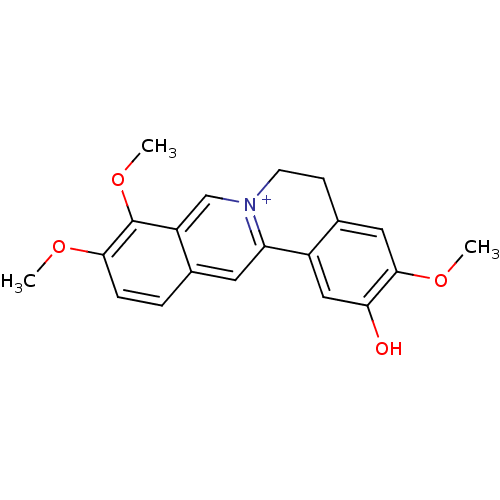
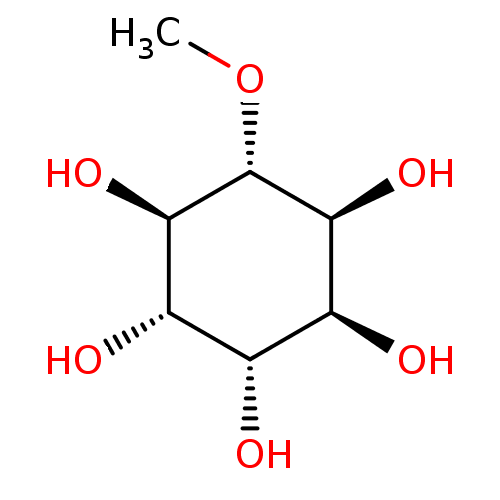
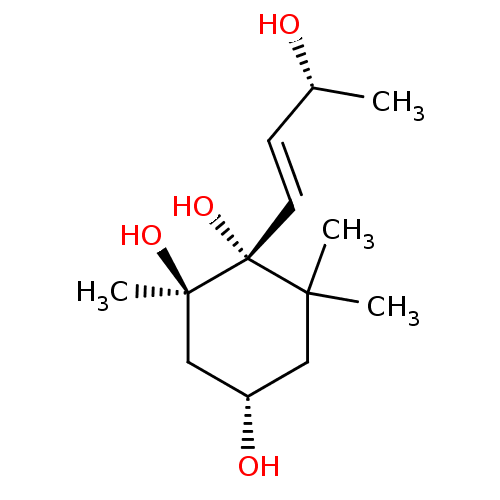
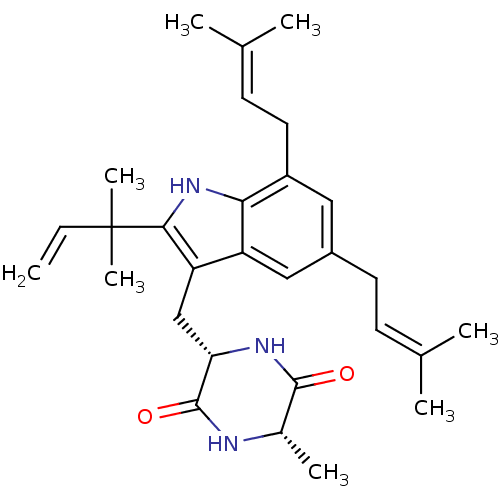
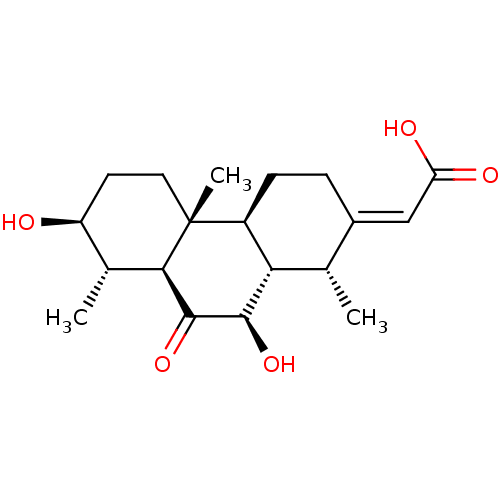
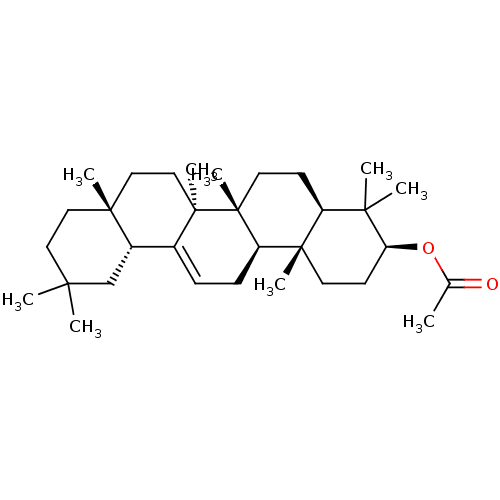
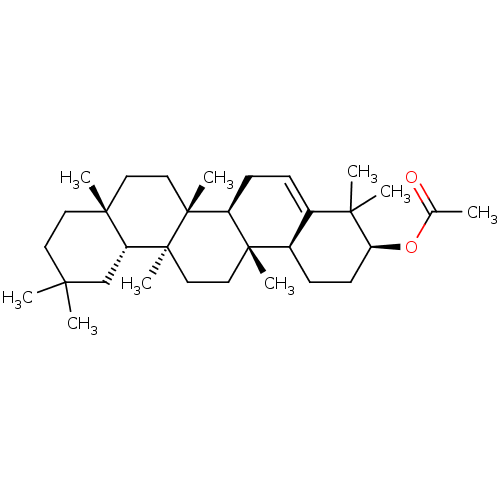
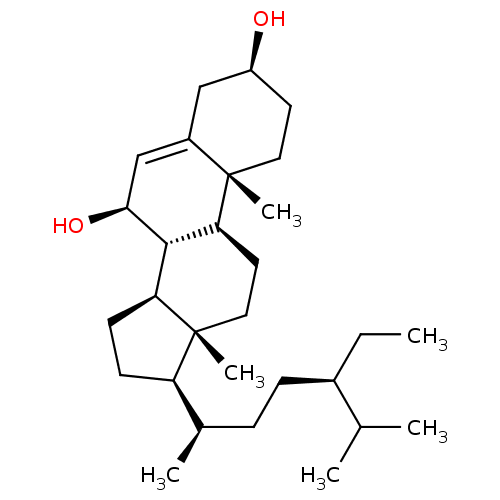
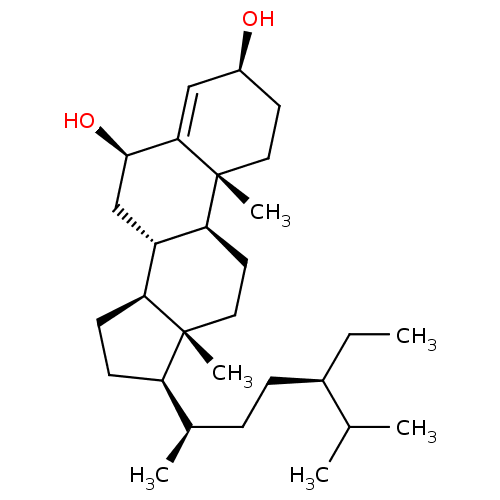
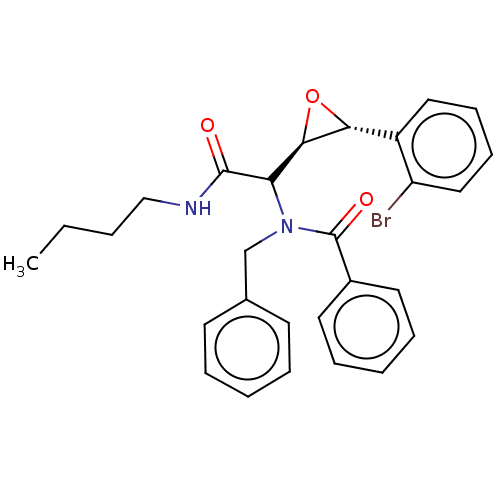
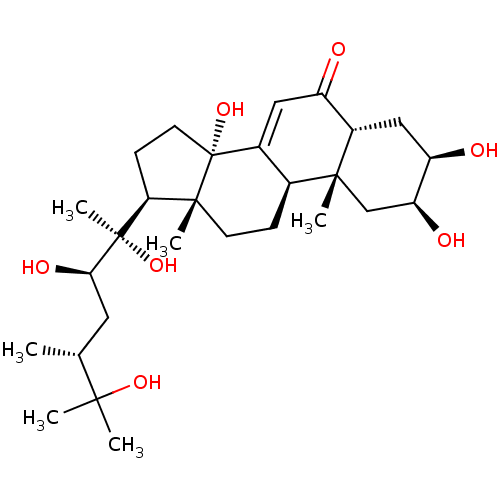
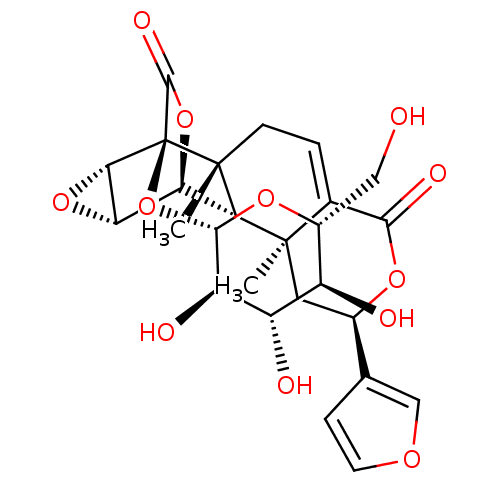
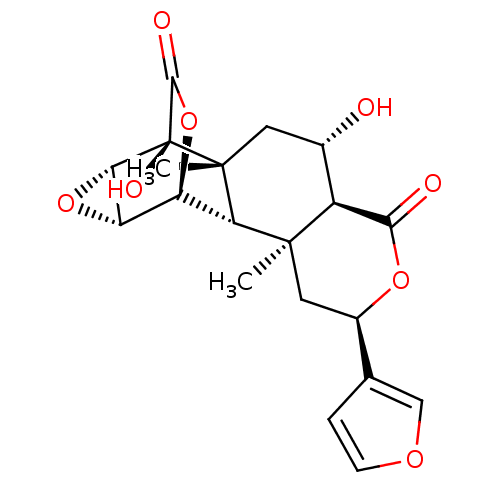
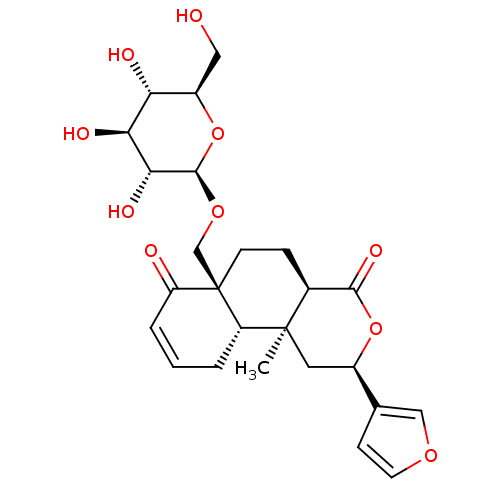
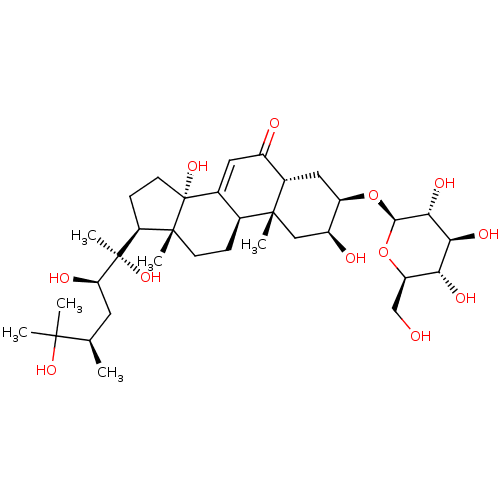
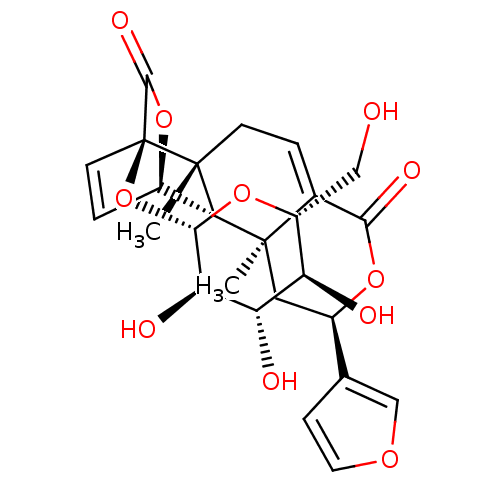
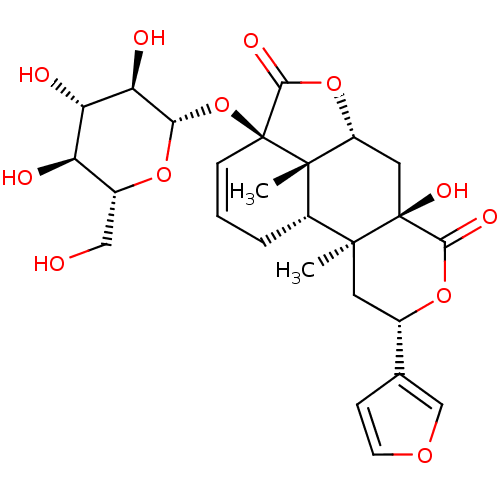
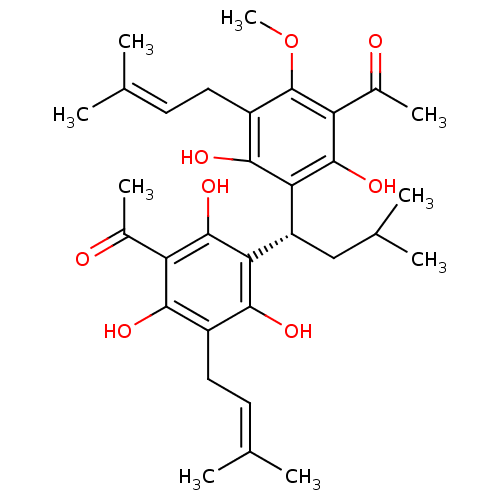
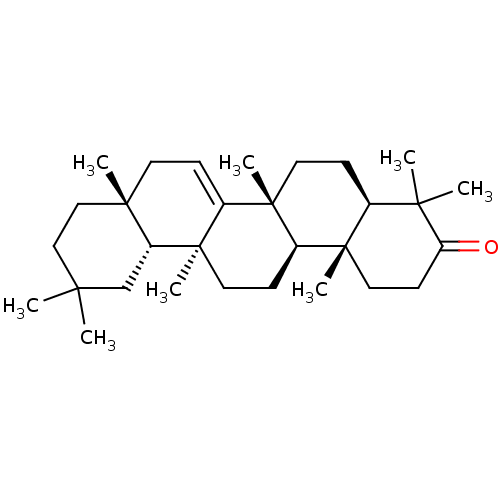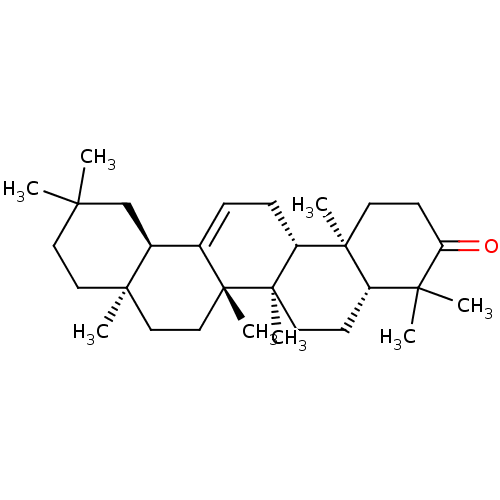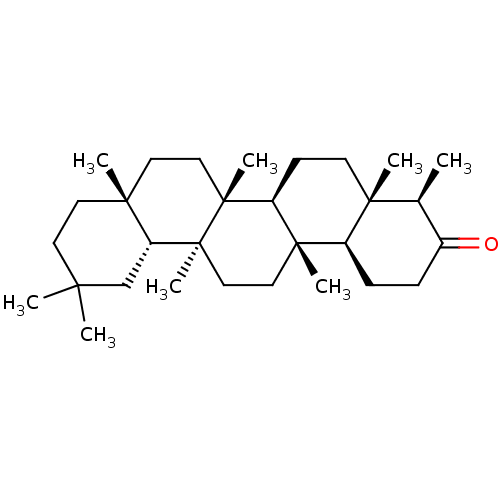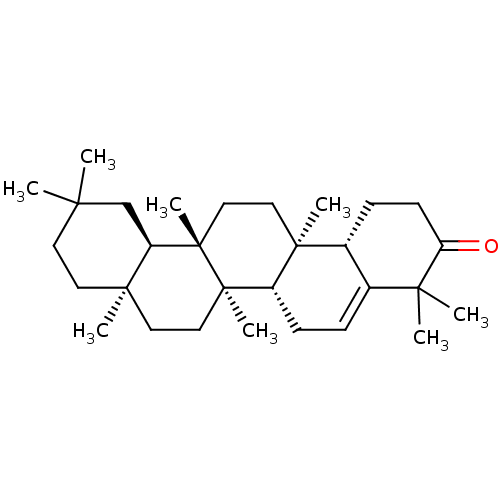Affinity DataKi: 5.45E+3nMAssay Description:Binding affinity to recombinant human cathepsin KMore data for this Ligand-Target Pair
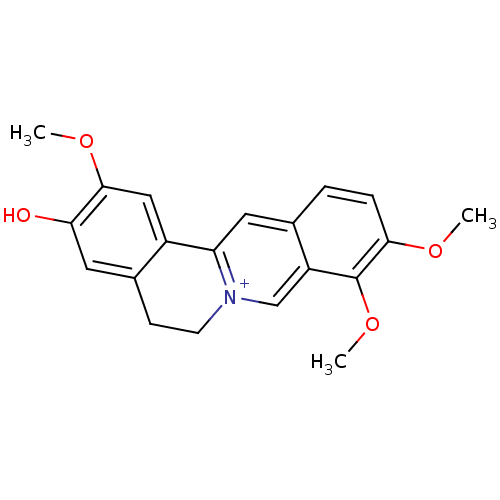
Affinity DataKi: 9.57E+3nMAssay Description:Binding affinity to recombinant human cathepsin KMore data for this Ligand-Target Pair
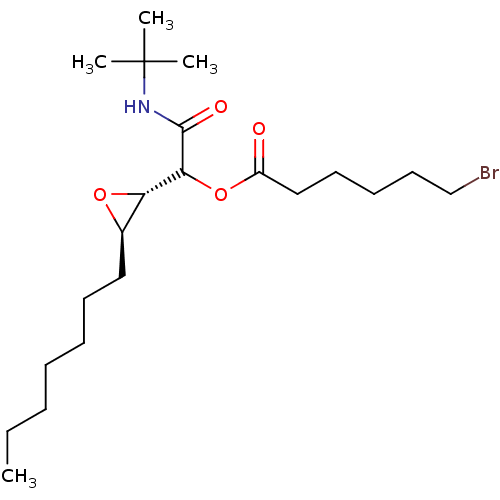
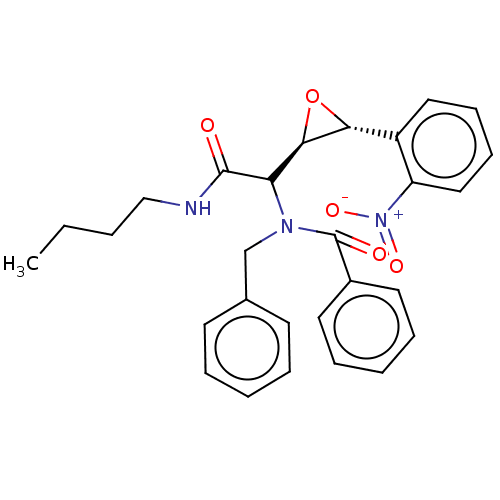
Affinity DataIC50: 900nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
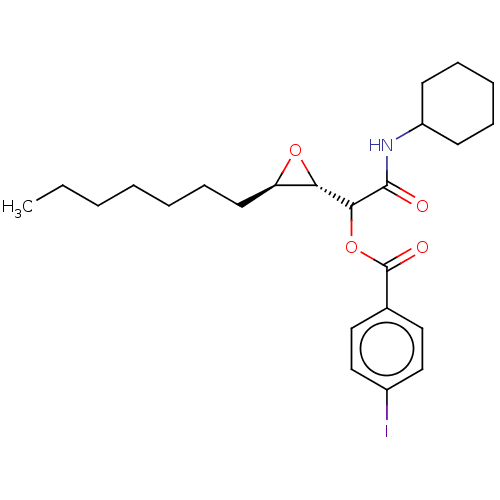
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.21E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 3.35E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.81E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
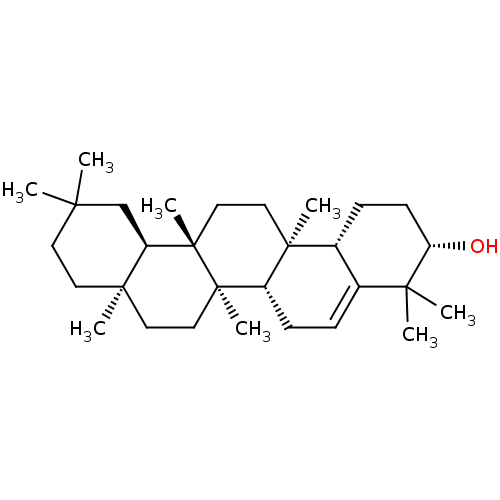
Affinity DataIC50: 5.36E+3nMAssay Description:Inhibition of cathepsin L (unknown origin) using fluorogenic Z-FR-MCA as substrate preincubated for 5 mins followed by substrate addition by spectrof...More data for this Ligand-Target Pair
Affinity DataIC50: 5.72E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 6.44E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of recombinant human cathepsin K using Z-Phe-Arg-MCA as fluorogenic substrate preincubated for 5 mins followed by substrate addition and m...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
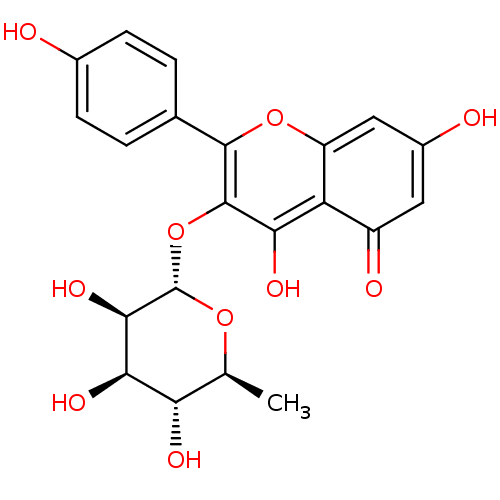
Affinity DataIC50: 2.42E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.58E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+4nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 4.65E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of cathepsin L (unknown origin) using fluorogenic Z-FR-MCA as substrate preincubated for 5 mins followed by substrate addition by spectrof...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of cathepsin L (unknown origin) using fluorogenic Z-FR-MCA as substrate preincubated for 5 mins followed by substrate addition by spectrof...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+5nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+5nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.33E+5nMAssay Description:Inhibition of tyrosinase (unknown origin)More data for this Ligand-Target Pair