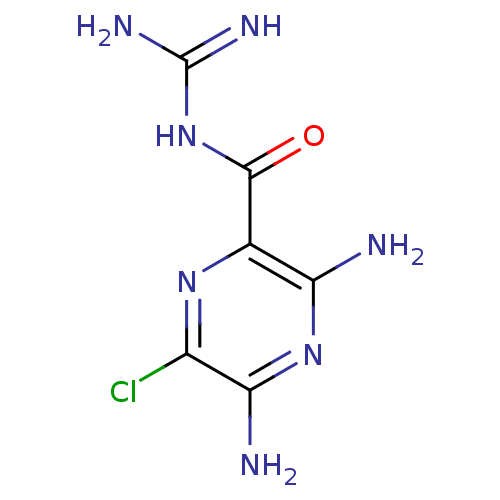
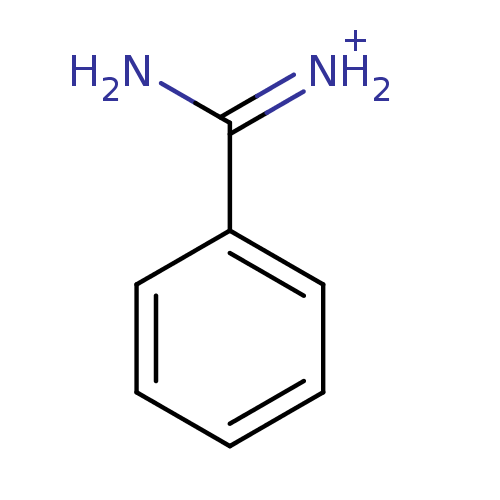
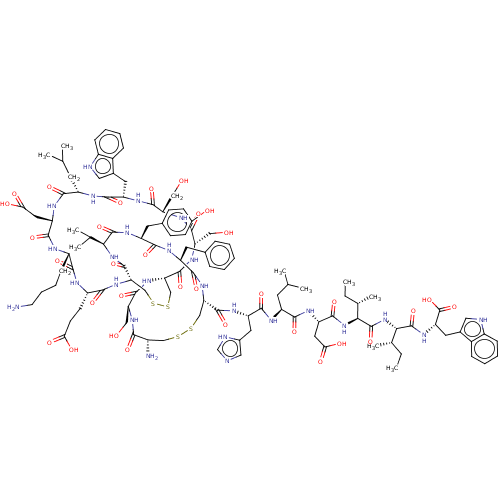
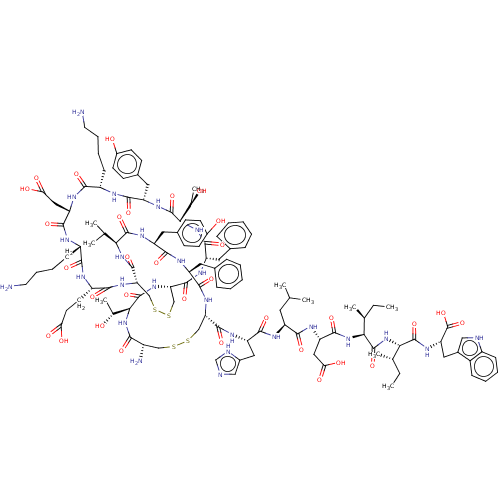
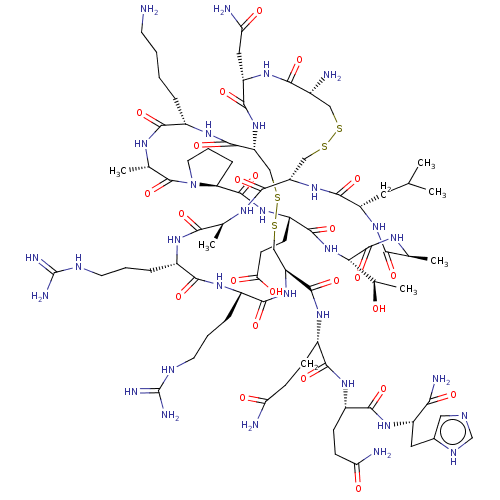
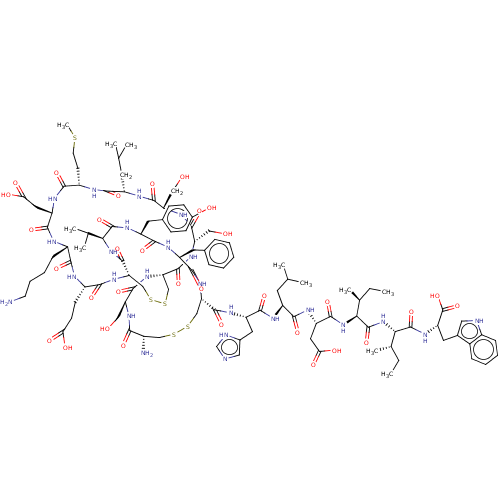
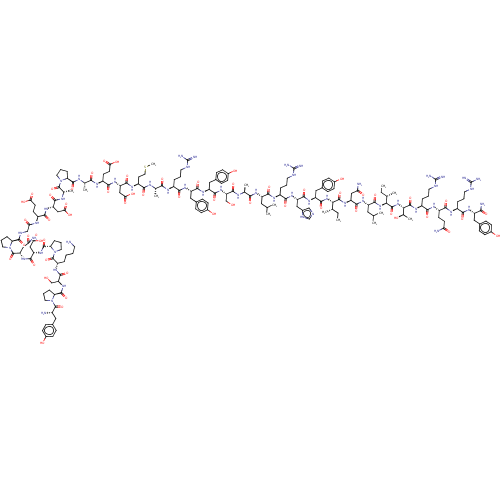
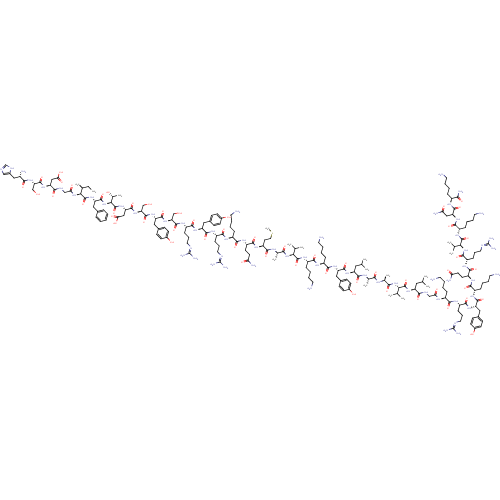
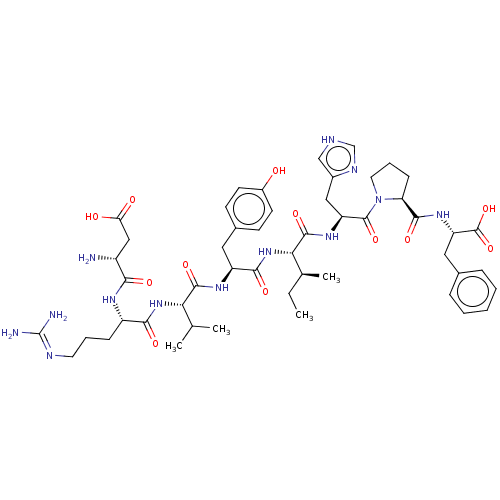
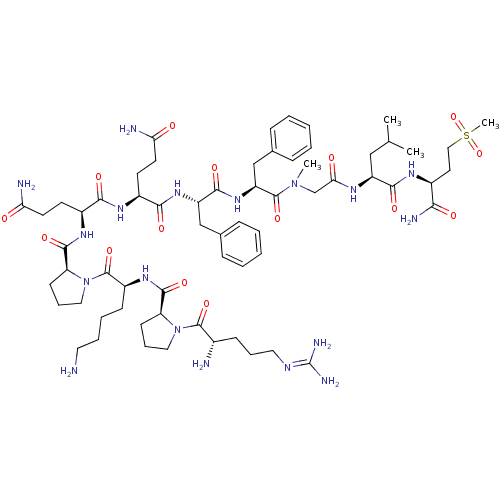
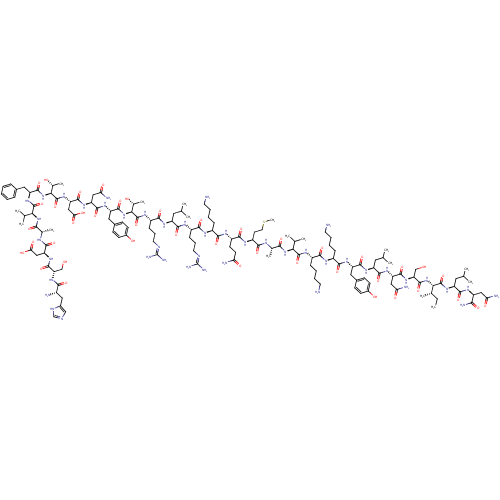
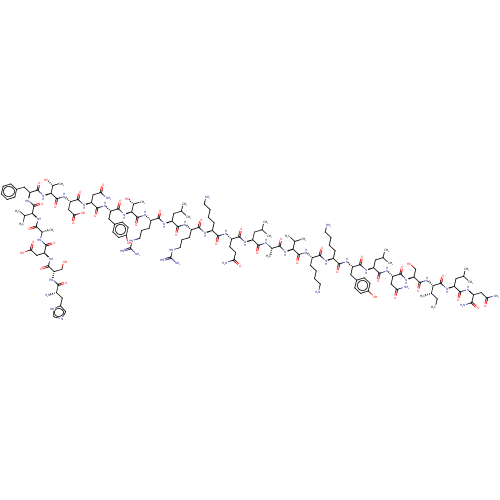
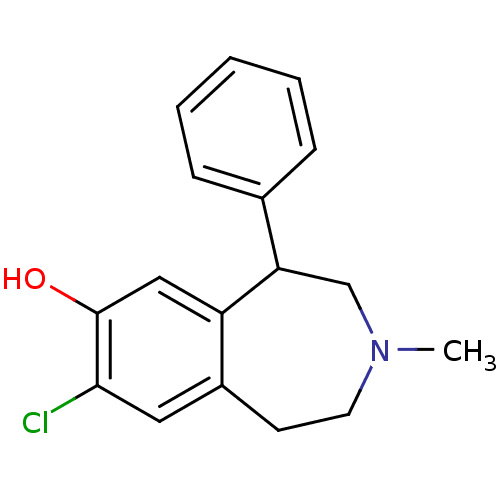
Affinity DataKi: 37nM ΔG°: -42.4kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
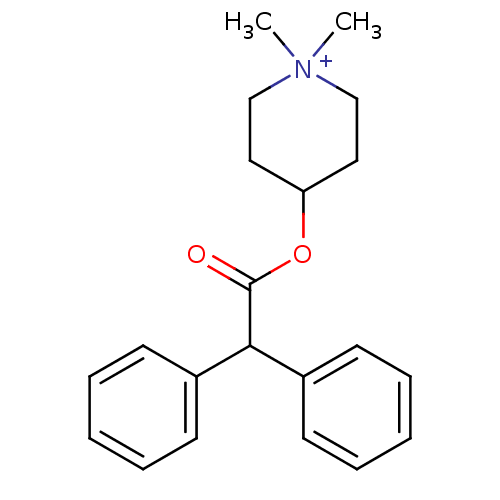
Affinity DataKi: 410nM ΔG°: -36.5kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 600nM ΔG°: -35.5kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 640nM ΔG°: -35.4kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nM ΔG°: -32.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 4.90E+3nM ΔG°: -30.3kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nM ΔG°: -30.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 8.70E+3nM ΔG°: -28.9kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nM ΔG°: -25.7kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 3.90E+4nM ΔG°: -25.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2445JRMPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2445JRMPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataKi: 4.60E+4nM ΔG°: -24.8kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+5nM ΔG°: -21.4kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nM ΔG°: >-17.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nM ΔG°: >-17.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nM ΔG°: >-17.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t...More data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.00600nMAssay Description:Displacement of [125I]Endothelin-1 from human recombinant Endothelin-B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.00600nMAssay Description:Displacement of [125I]endothelin-1 from human recombinant ETB receptor expressed in CHO cells measured after 120 mins by scintillation counting metho...More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0140nMAssay Description:Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSmall conductance calcium-activated potassium channel protein 1/2/3(Rattus norvegicus)
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0160nMAssay Description:Displacement of [125I]apamin from rat cerebral cortex small conductance calcium-activated potassium channelMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0230nMAssay Description:Displacement of [125I]endothelin-1 from human recombinant ETA receptor expressed in CHO cells measured after 120 mins by scintillation counting metho...More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0230nMAssay Description:Displacement of [125I]Endothelin-1 from human recombinant Endothelin-1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0280nMAssay Description:Displacement of [125I]peptide YY from Y2 receptor in human KAN-TS cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0320nMAssay Description:Displacement of [125I]hCGRPa from human recombinant CGRP receptor expressed in CHO cells measured after 90 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetVasoactive intestinal polypeptide receptor 1(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0400nMAssay Description:Displacement of [125I]PACAP1-27 from human recombinant PAC1 receptor expressed in CHO cells measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1B(Rattus norvegicus)
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0740nMAssay Description:Displacement of [125I]Tyr11-somatostatin-14 from somatostatin receptor in mouse AtT20 cellsMore data for this Ligand-Target Pair
TargetGalanin receptor type 1(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0790nMAssay Description:Displacement of [125I]galanin from human recombinant GAL1 receptor expressed in HEK293 cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetType-2 angiotensin II receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0800nMAssay Description:Displacement of [125I]CGP 42112A from human recombinant AT2 receptor expressed in HEK293 cells measured after 4 hrs by scintillation counting methodMore data for this Ligand-Target Pair
TargetType-2 angiotensin II receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.0800nMAssay Description:Displacement of [125I]CGP42112A from human recombinant AT2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]2-iodomelatonin from human recombinant MT1 receptor expressed in CHO cells measured after 60 mins by scintillation counting met...More data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]BH-SP from NK1 receptor in human U373MG cellsMore data for this Ligand-Target Pair
TargetVasoactive intestinal polypeptide receptor 1(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]VIP from human recombinant VIP1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMelatonin receptor type 1A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]2-iodomelatonin from human recombinant ML1A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]BH-SP from NK1 receptor in human U373MG cells measured after 30 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetVasoactive intestinal polypeptide receptor 1(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]VIP from human recombinant VPAC1 receptor expressed in CHO cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Displacement of [125I]CCK-8s from human recombinant CCK-B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Displacement of [125I]CCK-8s from human recombinant CCK2 receptor expressed in CHO cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Displacement of [125I]CCK-8s from human recombinant CCK1 receptor expressed in CHO cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Displacement of [125I]CCK-8s from human recombinant CCK-A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMelanocortin receptor 4(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Displacement of [125I]NDP-a-MSH from human recombinant MC4 receptor expressed in CHO cells measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetMelanocortin receptor 4(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Displacement of [125I]NDP-alpha-MSH from human recombinant MC4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Displacement of [125I]peptide YY from Y1 receptor in human SK-N-MC cells measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells measured after 120 mins by scintillation counting methodMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetD(1A) dopamine receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:Displacement of [3H]SCH23390 from human recombinant Dopamine D1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetD(1A) dopamine receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:Displacement of [3H]SCH 23390 from human recombinant dopamine D1 receptor expressed in CHO cells measured after 60 mins by scintillation counting met...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Displacement of [3H]4-DAMP from human recombinant muscarinic M5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Displacement of [3H]4-DAMP from human recombinant M5 receptor expressed in CHO cells measured after 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataIC50: 0.350nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair