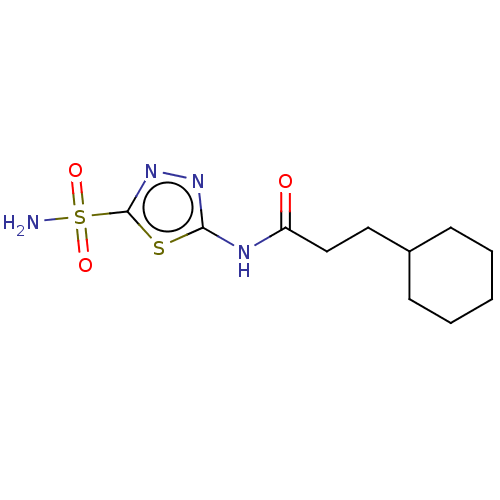
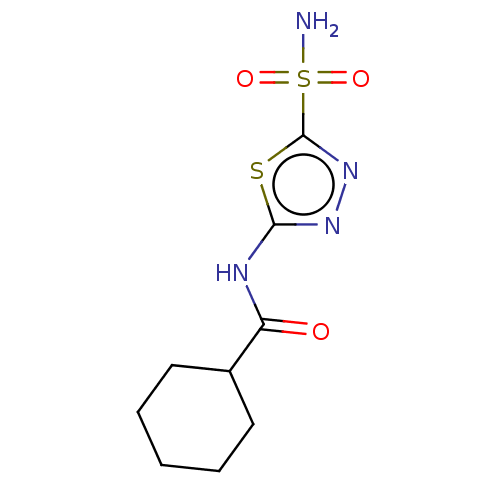
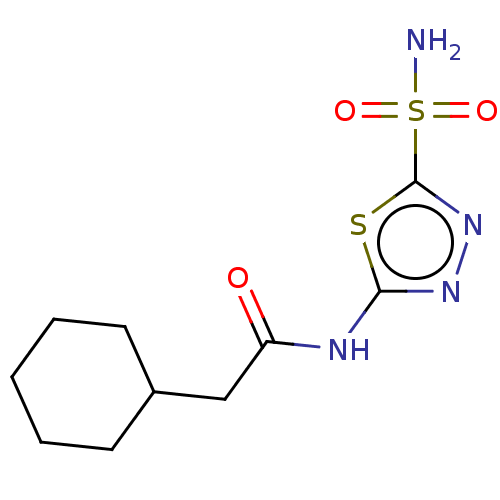
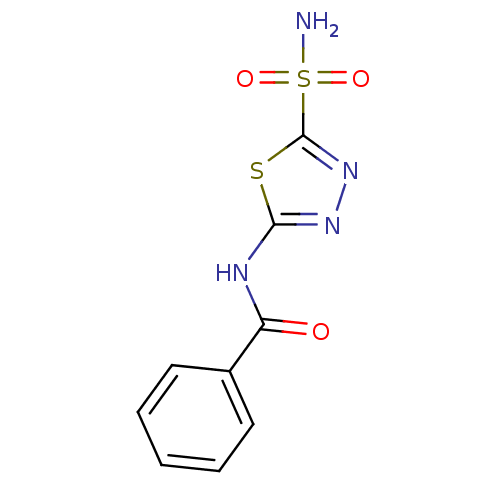
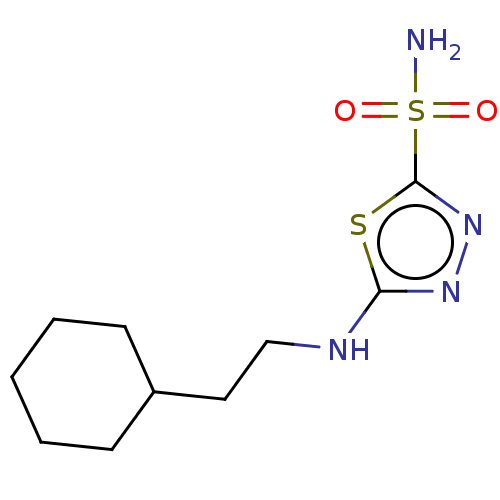
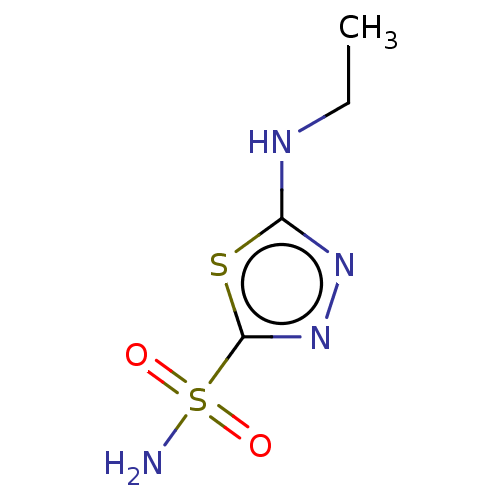
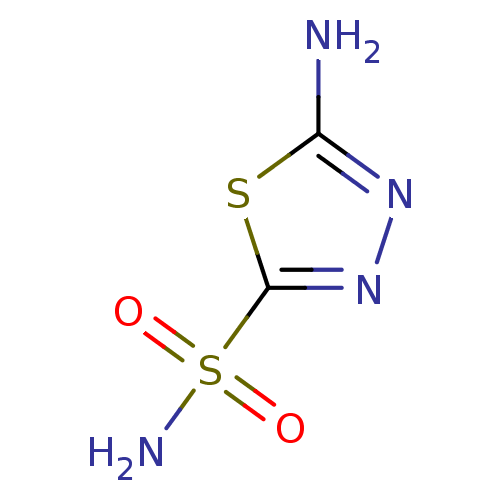
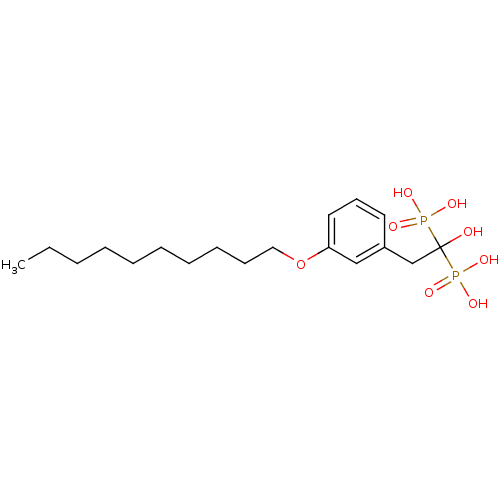
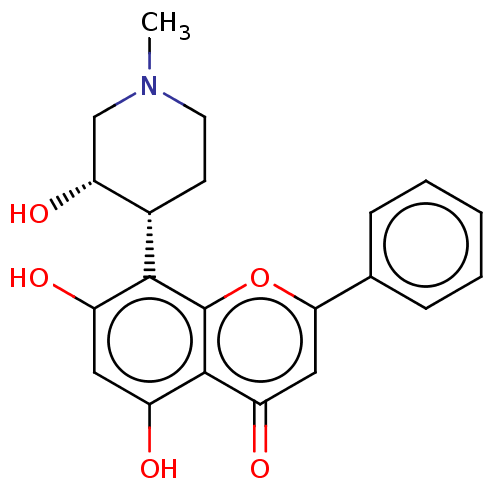
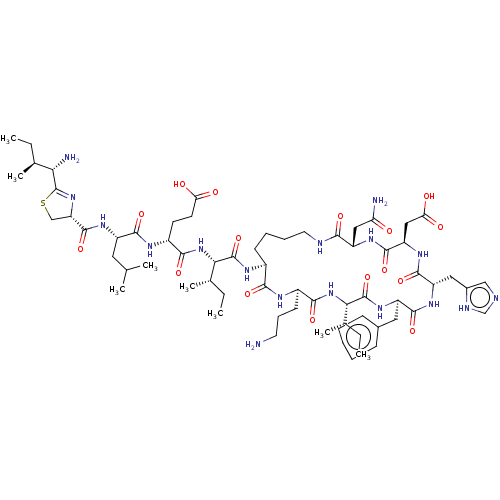
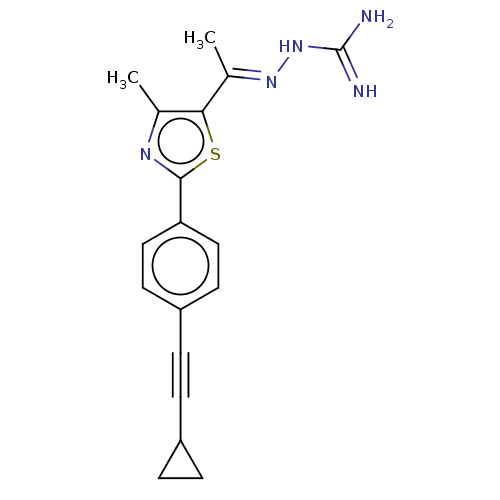
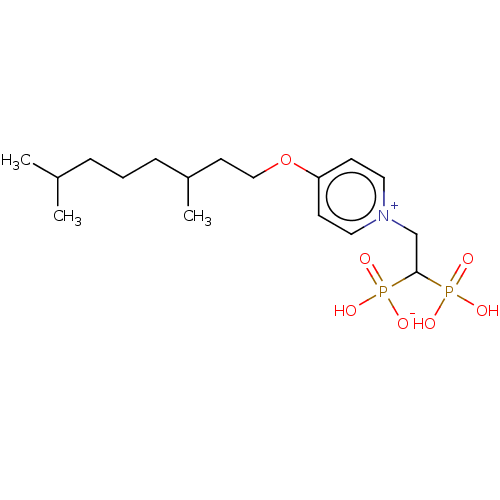
Affinity DataKi: 0.320nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 8.10nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 13nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of human carbonic anhydrase 13 expressed in Escherichia coliBL21(DE3) pLysS incubated for 10 mins by phenol red dye based assayMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 29nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 38nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 47nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 60nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Antihistaminic activity against Histamine H1 receptor was measured on isolated terminal part of guinea pig ileumMore data for this Ligand-Target Pair
In DepthDetails
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human FPPS using IPP and GPP as substrateMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2S75KV9PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2S75KV9PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of human CDK2 expressed in sf9 insect cellsMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human CDK2 expressed in sf9 insect cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of Escherichia coli UPPP expressed in Escherichia coli C41(DE3) using FPP as substrate preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of Escherichia coli UPPP expressed in Escherichia coli C41(DE3) using FPP as substrate preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Affinity DataIC50: 3.33E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
TargetDitrans,polycis-undecaprenyl-diphosphate synthase ((2E,6E)-farnesyl-diphosphate specific)(Escherichia coli (strain K12))
Al-Azhar University
Curated by ChEMBL
Al-Azhar University
Curated by ChEMBL
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of Escherichia coli UPPS using IPP and FPP as substrate preincubated for 30 mins followed by substrate addition measured after 20 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.32E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 5.74E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 6.32E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
TargetDitrans,polycis-undecaprenyl-diphosphate synthase ((2E,6E)-farnesyl-diphosphate specific)(Escherichia coli (strain K12))
Al-Azhar University
Curated by ChEMBL
Al-Azhar University
Curated by ChEMBL
Affinity DataIC50: 6.60E+4nMAssay Description:Inhibition of Escherichia coli UPPS in presence of IPP and FPPMore data for this Ligand-Target Pair
Affinity DataIC50: 8.81E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human FPPS using IPP and GPP as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
Affinity DataIC50: 3.55E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis PPase using inorganic pyrophosphate as substrate preincubated for 2 mins followed by substrate addition meas...More data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.50E+5nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair