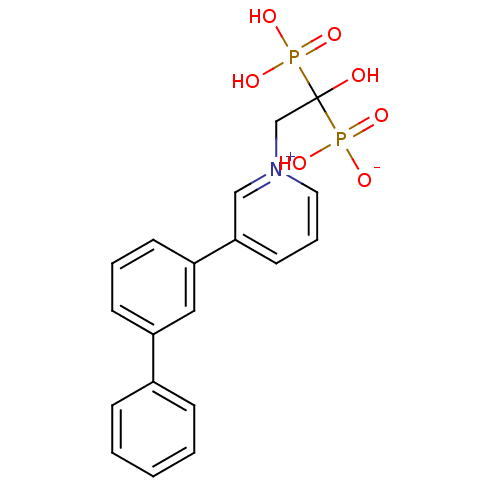
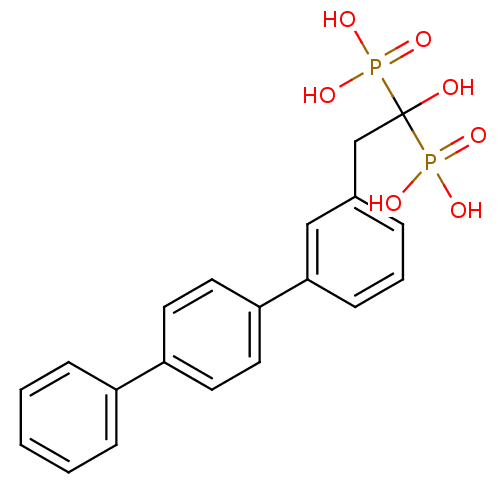
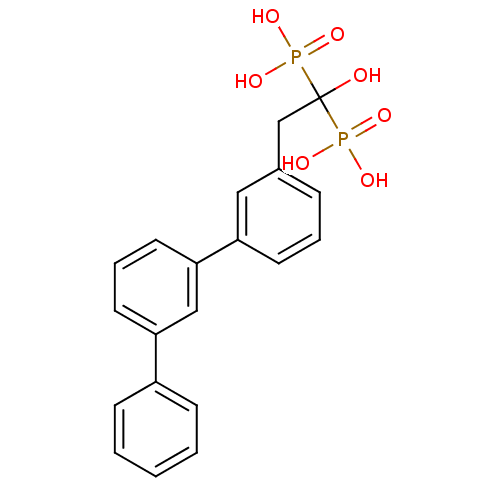
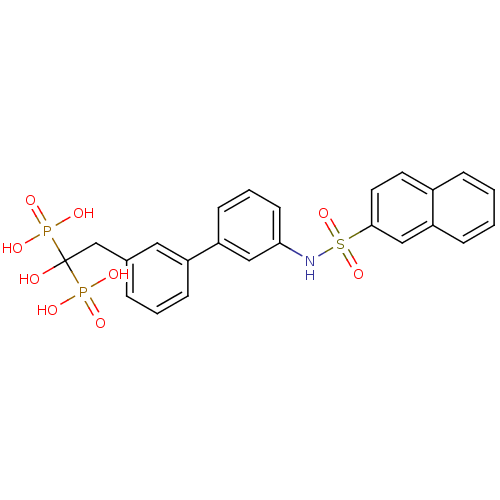
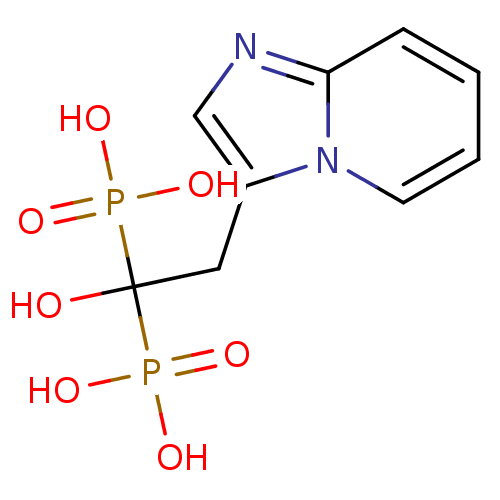
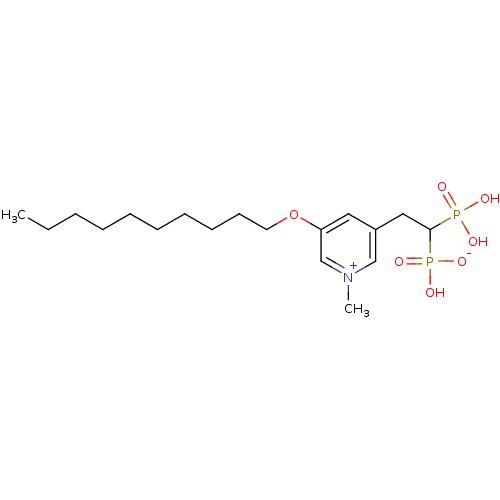
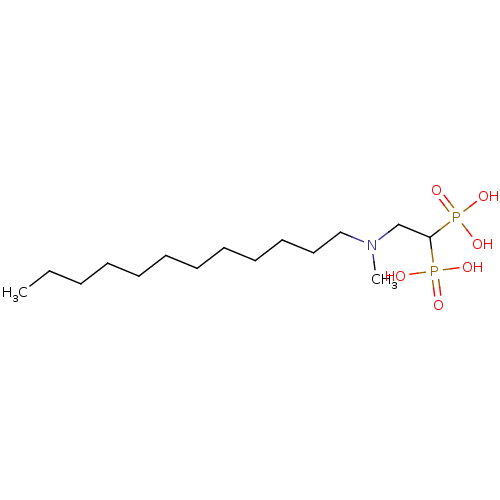
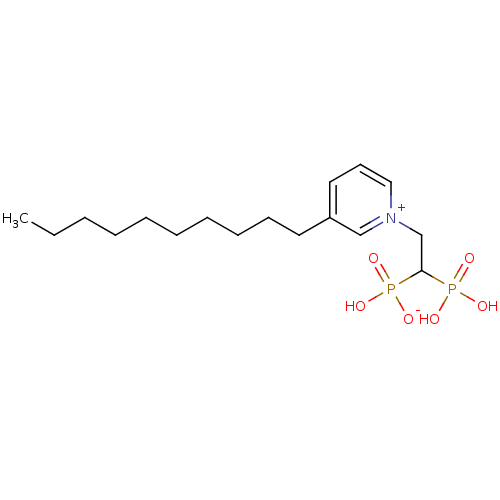
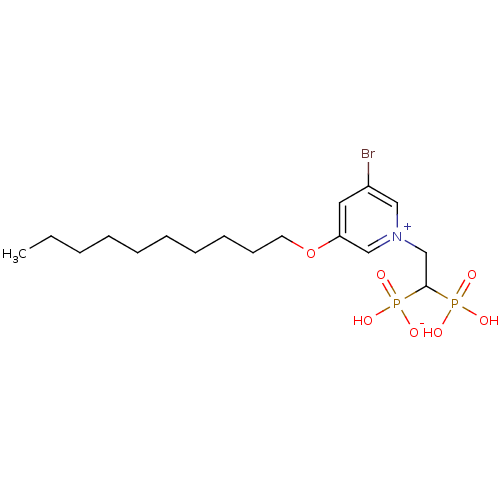
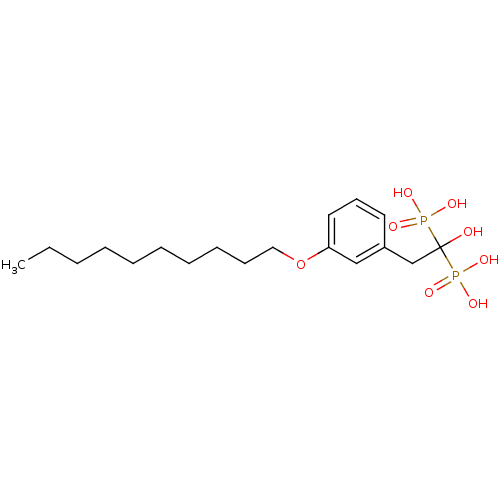
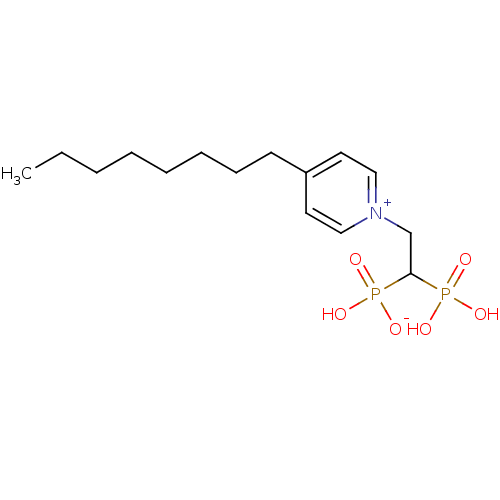
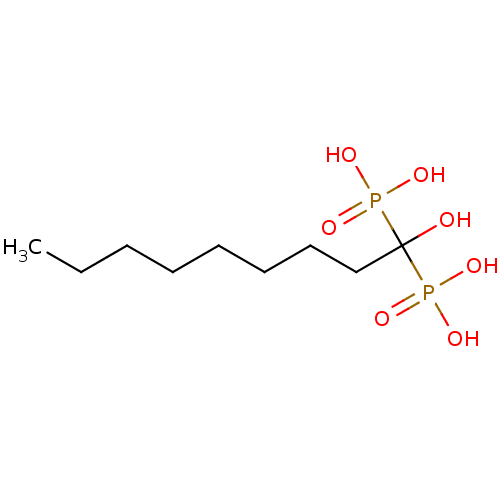
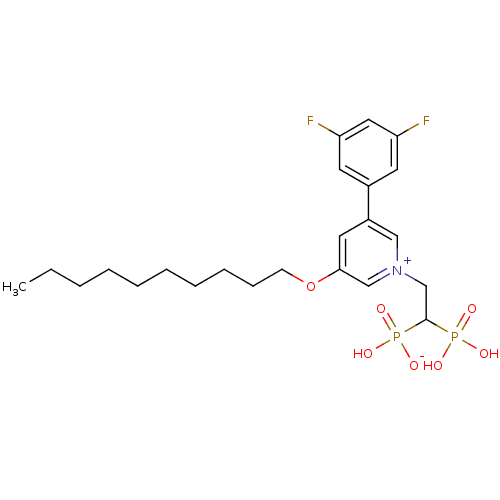
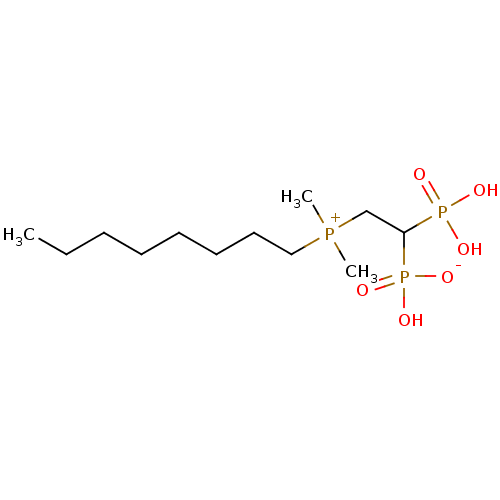
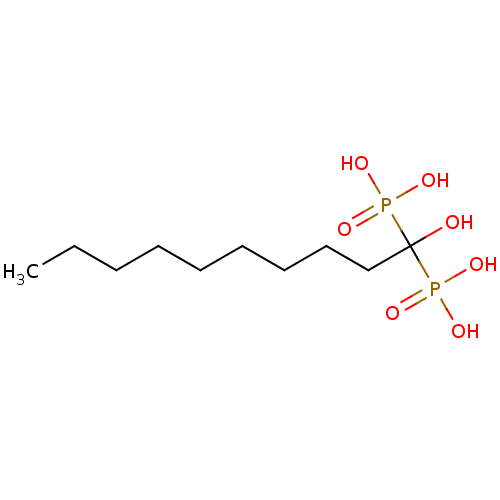
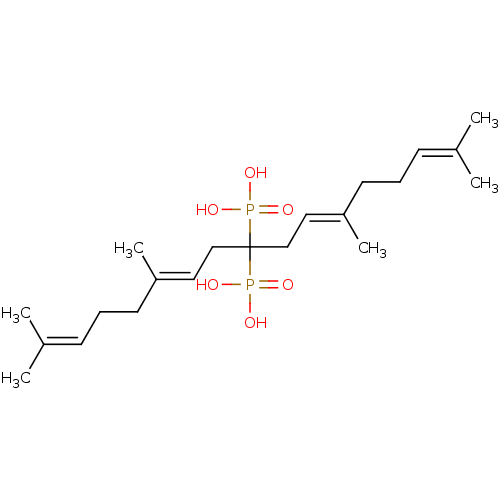
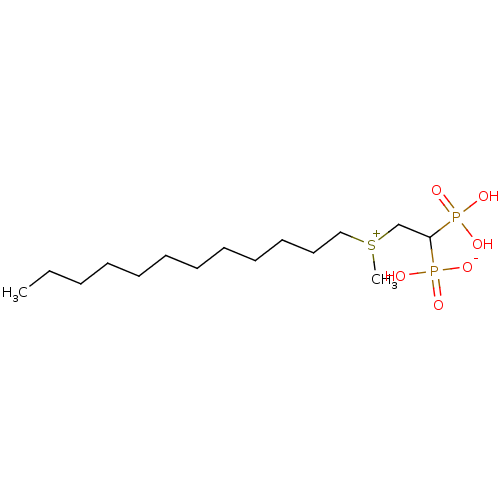
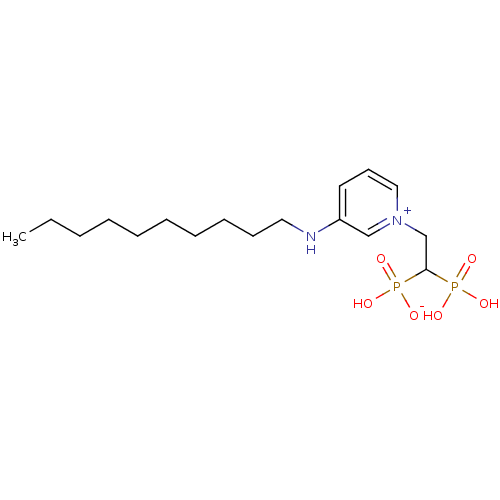
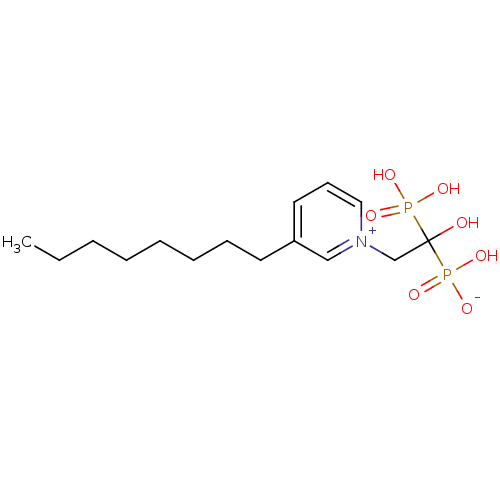
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Binding affinity to Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 2.70E+3nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 100nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human FPPS using IPP and GPP as substrateMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
Chinese Academy of Sciences
Curated by ChEMBL
Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human FPPS using IPP and GPPMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2S75KV9PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2S75KV9PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 280nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 280nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 350nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 400nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 510nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 590nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase BTS1(Saccharomyces cerevisiae (Yeast))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 660nMAssay Description:Inhibition of Saccharomyces cerevisiae GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 660nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 710nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 720nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 720nMAssay Description:Inhibition of human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 760nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 890nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 890nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 980nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 1.15E+3nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 1.23E+3nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 1.26E+3nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 1.38E+3nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute of Biological Chemistry
Curated by ChEMBL
Institute of Biological Chemistry
Curated by ChEMBL
Affinity DataIC50: 1.48E+3nMpH: 7.0 T: 2°CAssay Description:The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co...More data for this Ligand-Target Pair