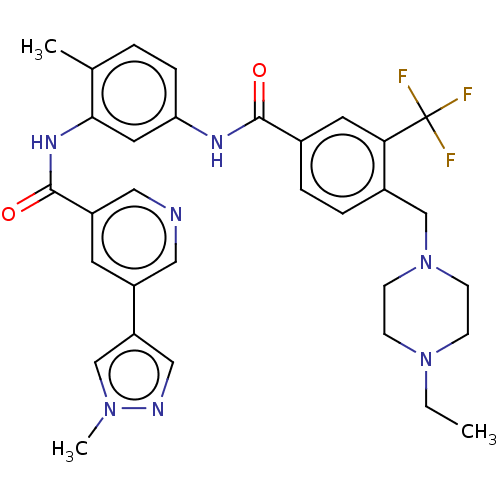
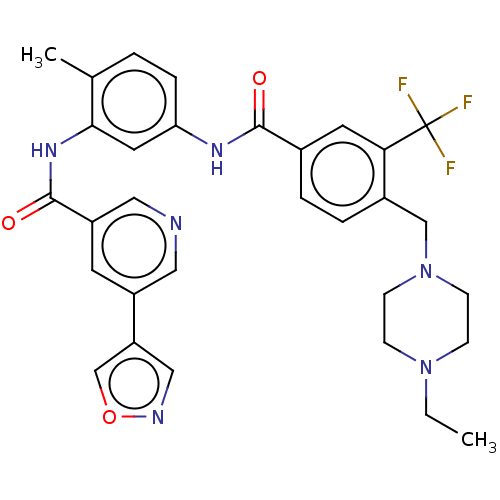
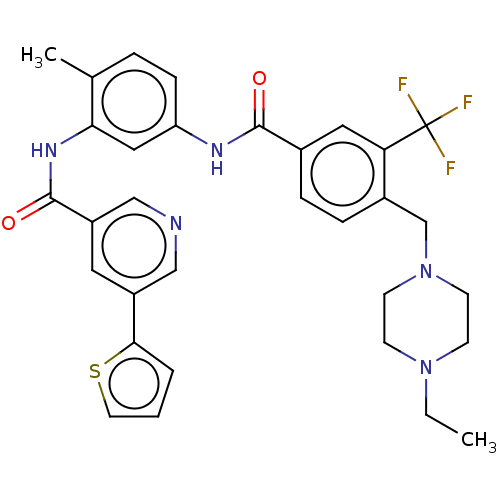
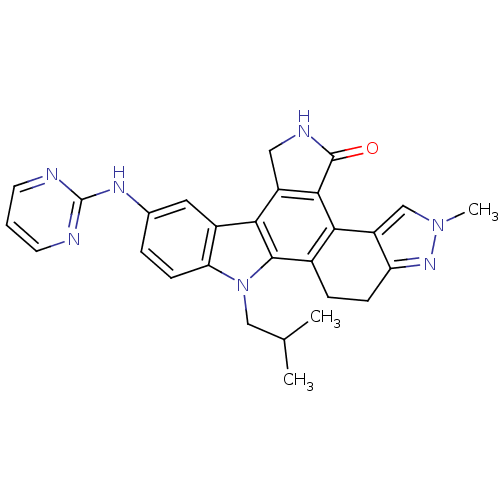
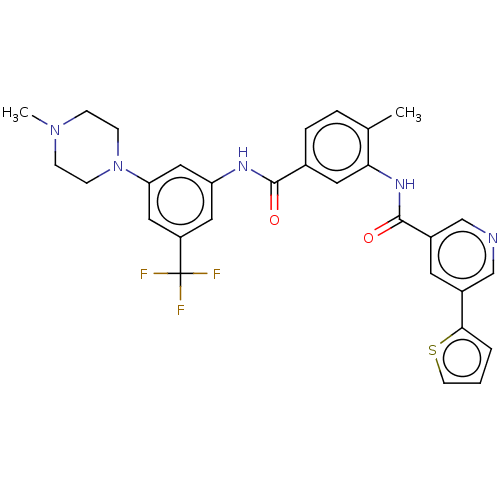
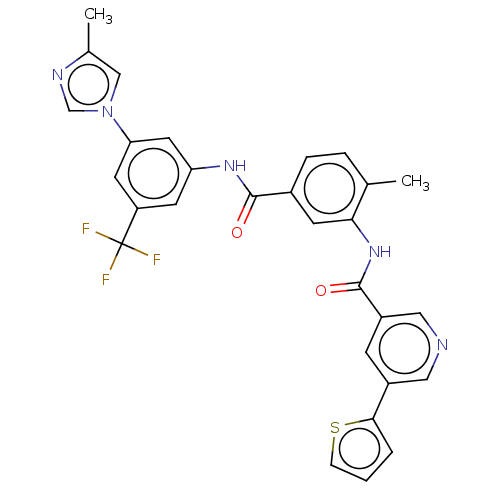
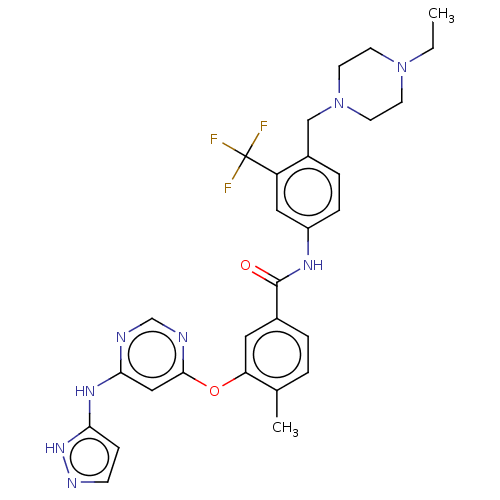
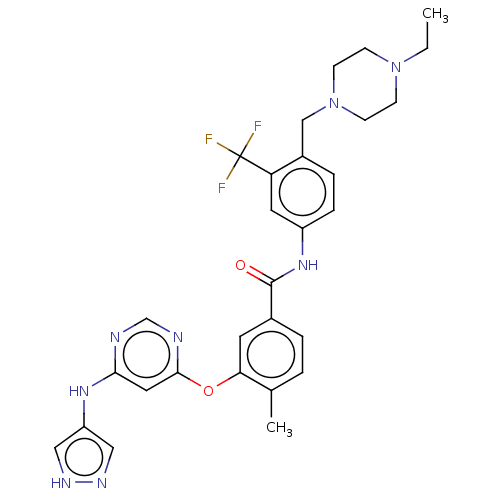
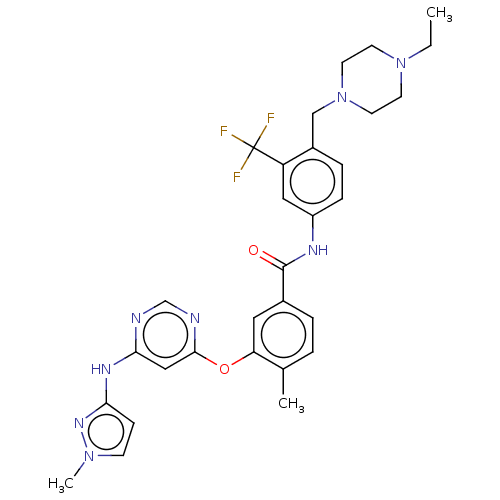
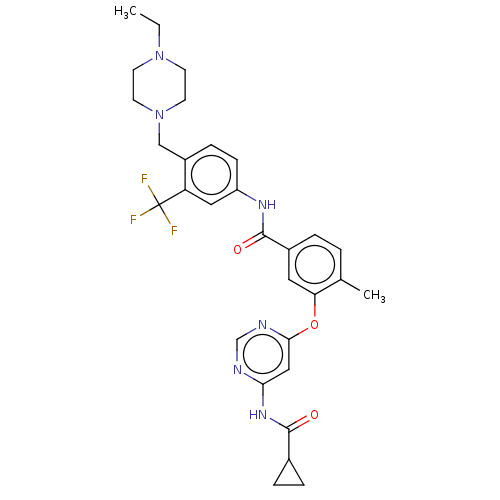
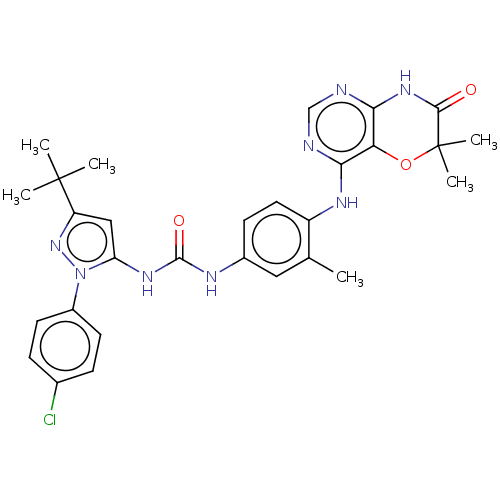
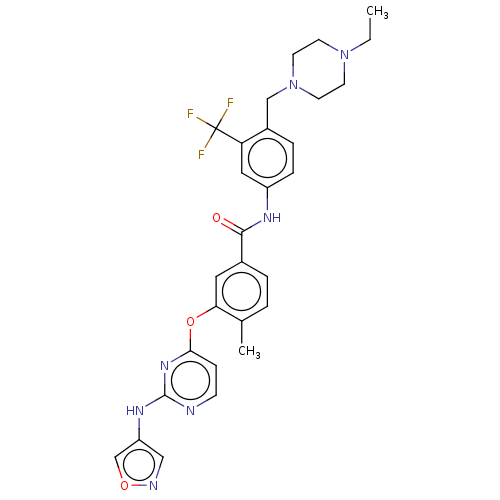
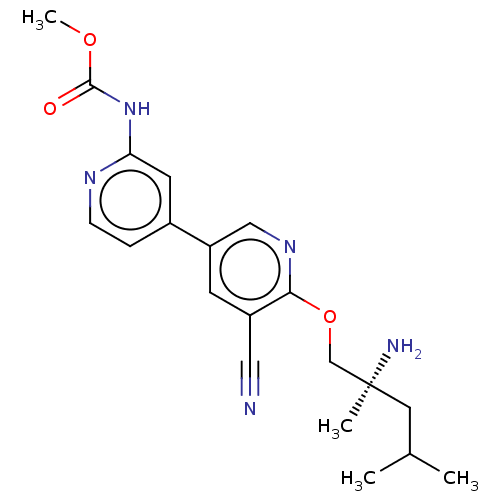
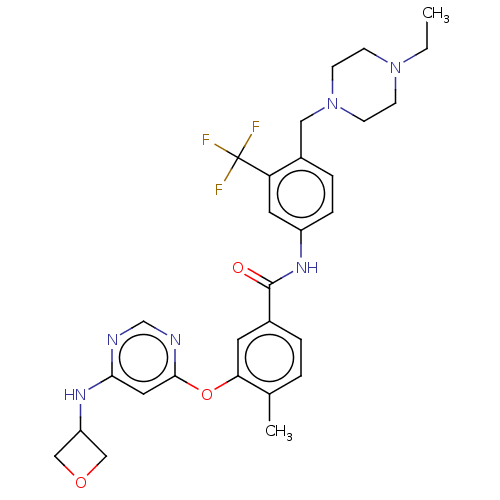
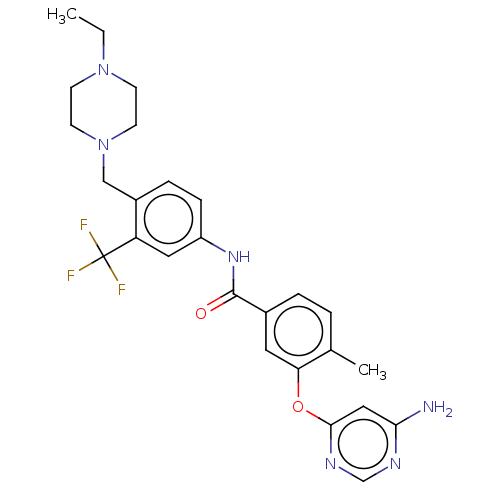
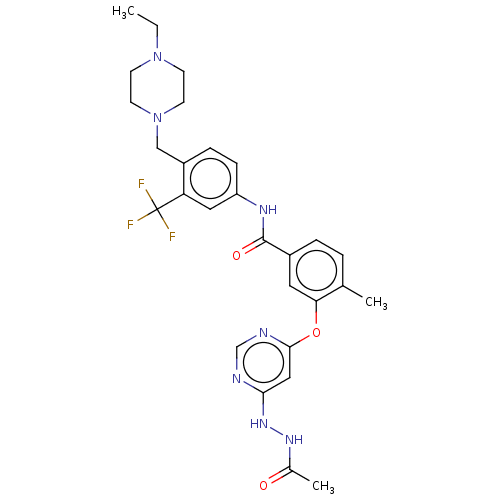
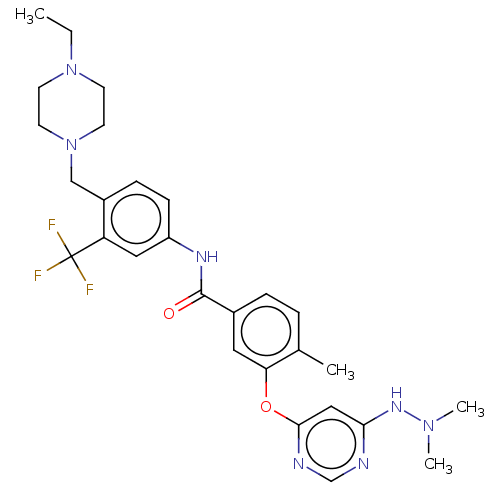
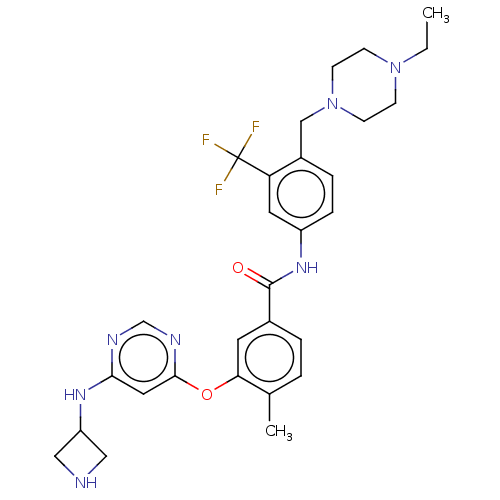
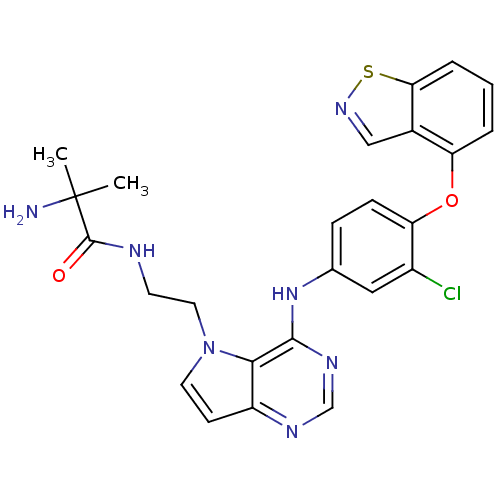
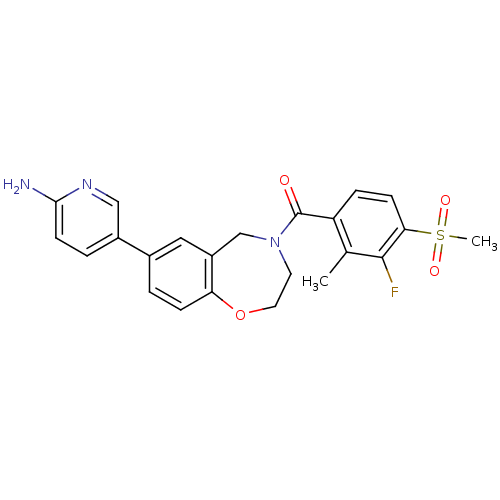
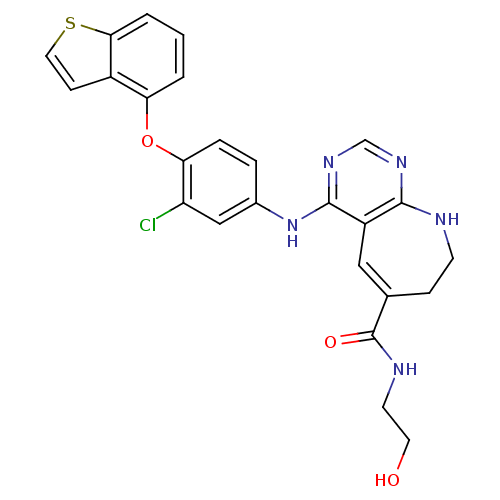
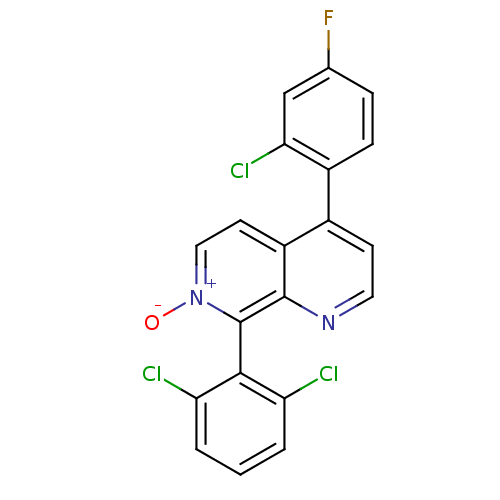
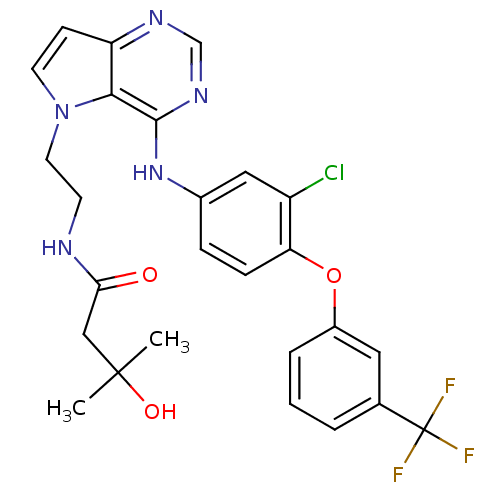
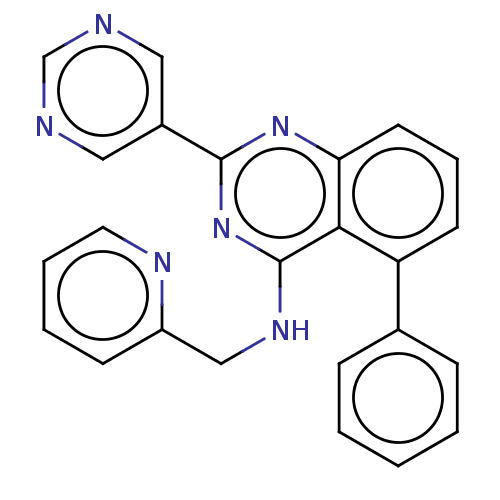
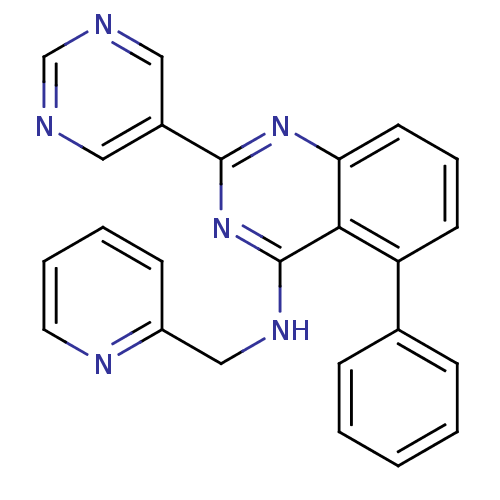
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 2.43nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
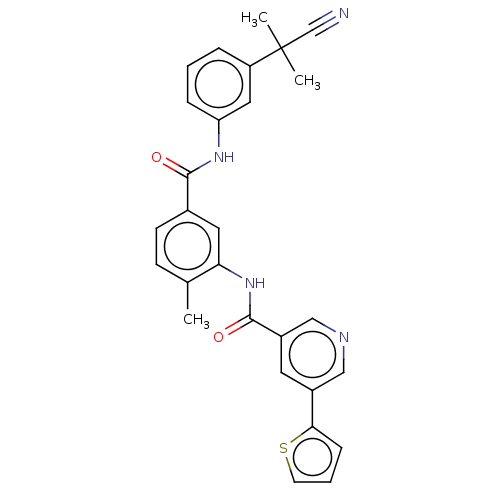
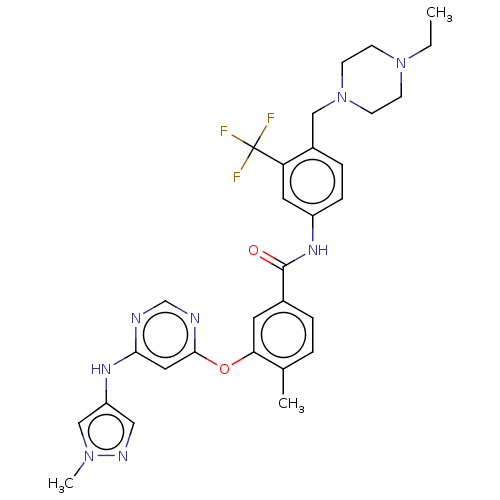
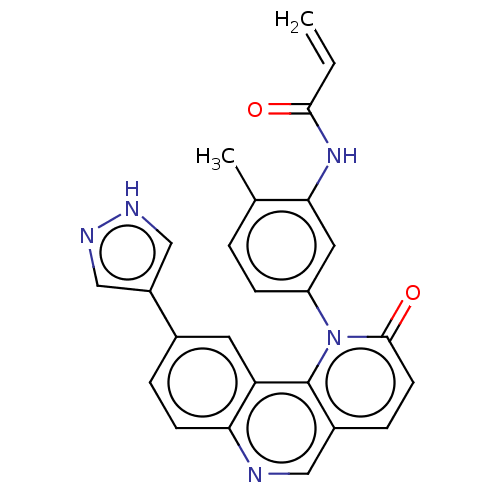
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 5.33nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
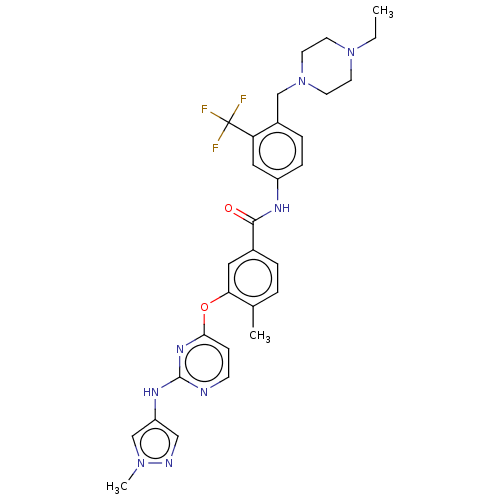
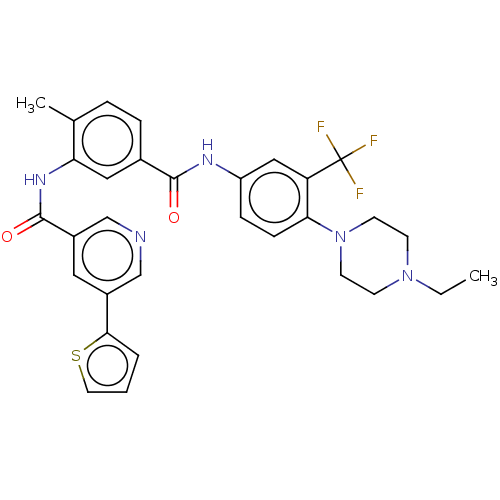
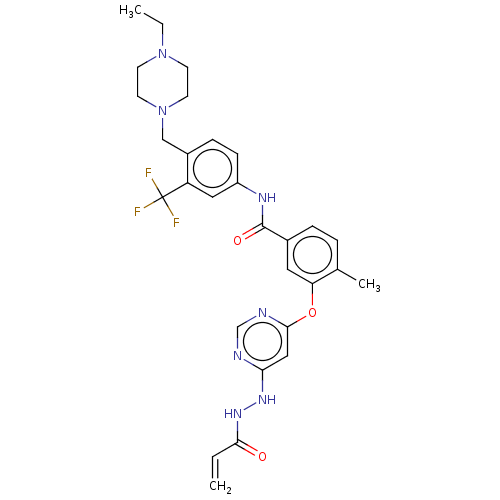
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 7.40nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
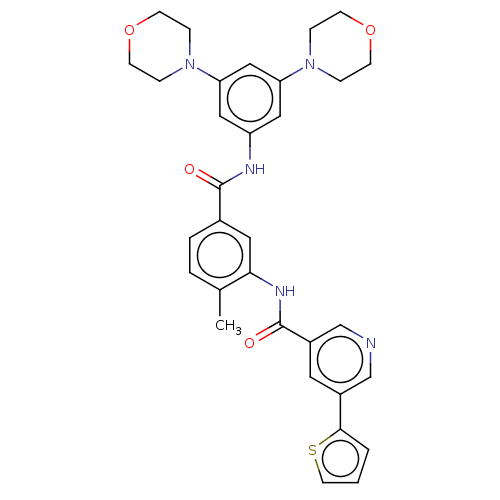
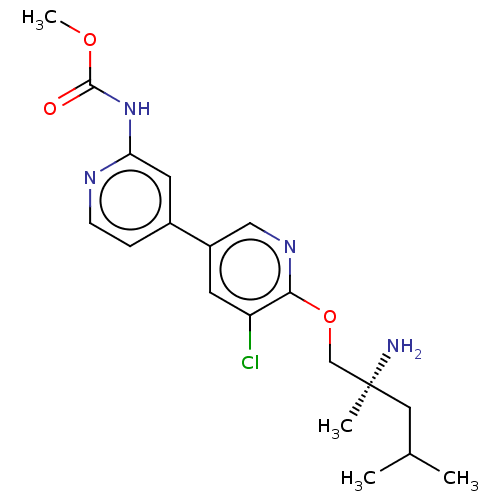
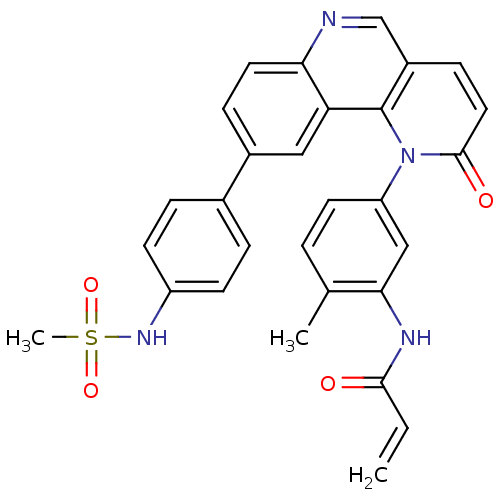
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 14nMAssay Description:Inhibition of human TAK1 using ATP as substrateMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 22.8nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 29.9nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 45nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 53.8nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 56.5nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 61nMAssay Description:Inhibition of human TAK1More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 62.2nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 63.5nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 71.7nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 76.2nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 92nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 100nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 100nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 117nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 126nMAssay Description:Inhibition of human TAK1 in presence of ATP by radiometric assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 136nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 364nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 400nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 487nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 591nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 717nMAssay Description:DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.75E+3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 3.30E+3nMAssay Description:DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 3.30E+3nMAssay Description:DiscoverX binding assays were performed according to published methods (Fabian et al., Nat. Biotechnol. 23, 329-36 (2005); Davis et al., Nat. Biotech...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 3.33E+3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 7.31E+3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 8.21E+3nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK and other kinases were obtained using an Invitrogen Select Screening ...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of N-terminus FLAG-tagged TAK1 expressed in baculovirus expression system using [gamma-33P]-ATP measured after 60 mins by scintillation co...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TAK1More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of TAK1 (unknown origin)More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of N-terminal FLAG-tagged TAK1 (unknown origin) expressed in baculovirus expression system incubated for 5 mins prior to [gamma-33P]ATP ad...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human TAK1More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of N-terminus flag-tagged TAK1 expressed in baculovirus infected insect cells using [gamma33P]ATP as substrate after 60 mins by scintillat...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 5.00E+4nMAssay Description:The analyses were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and subst...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group C member 2(Homo sapiens (Human))
The Scripps Research Institute
The Scripps Research Institute
Affinity DataIC50: 5.00E+4nMAssay Description:The analyses were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and subst...More data for this Ligand-Target Pair