

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1C(=O)C(=O)c2ccccc12
InChI Key: InChIKey=VCYBVWFTGAZHGH-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer. 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM10304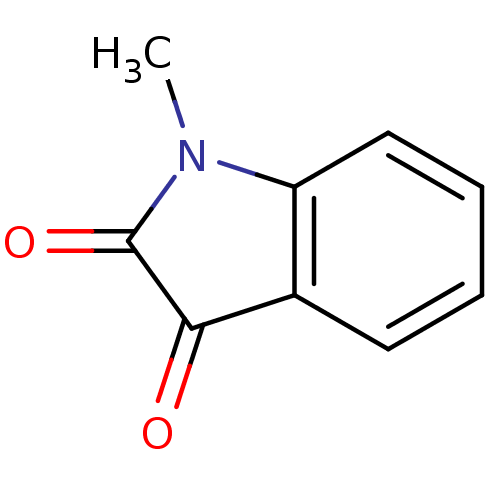 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.82E+4 | -6.02 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of TTR mediated fibrillogenesis assessed as acid-induced protein aggregation turbidity after 1.5 hrs by turbidimetric assay | Bioorg Med Chem Lett 19: 5270-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.004 BindingDB Entry DOI: 10.7270/Q2SF2W7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Secreted chorismate mutase (Mycobacterium tuberculosis H37Rv) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Birla Institute of Technology & Science-Pilani, Hyderabad Campus, Shameerpet, R.R. District, Hyderabad, Andhra Pradesh, 500078, India | Assay Description The reaction was initiated by the addition of 150pmoles of enzyme to a mixture of inhibitor (40µM, 20 µM, 10 µM, 5 µM, 1 µM ... | Chem Biol Drug Des 83: 498-506 (2014) Article DOI: 10.1111/cbdd.12265 BindingDB Entry DOI: 10.7270/Q2F76B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10304 (1-methyl-2,3-dihydro-1H-indole-2,3-dione | CHEMBL6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||