

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM285626 3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,2-b]pyridazine-2-carboxamide::US10077269, Example 96::US10669279, Example 96::US9598421, Example 96
SMILES: CCNC(=O)c1nc2cccnn2c1-c1cc(F)c(Cl)cc1F
InChI Key: InChIKey=IMOBPVDSTURWHO-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A Isoform 4 (Homo sapiens (Human)) | BDBM285626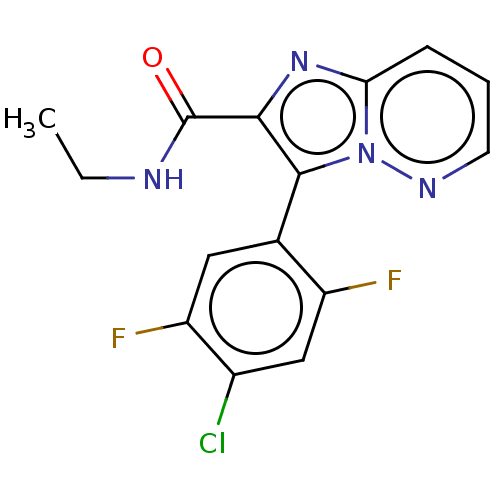 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C isoform 1 (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 908 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4A (PDE4) (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 908 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 908 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM285626 (3-(4-chloro-2,5-difluoro-phenyl)-N-ethylimidazo[1,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||