

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM295058 US10112926, Example 12::trans 3-[6-Amino-5-(3-methyl-[1,2,4]oxadiazol-5-yl)-pyridin-3-yl]-N-(4-hydroxy-cyclohexyl)-4-methyl-benzenesulfonamide, hydrochloride salt
SMILES: Cc1noc(n1)-c1cc(cnc1N)-c1cc(ccc1C)S(=O)(=O)N[C@H]1CC[C@H](O)CC1
InChI Key: InChIKey=XFGGNFBJRMXYAG-WKILWMFISA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI3-kinase class I (Homo sapiens (Human)) | BDBM295058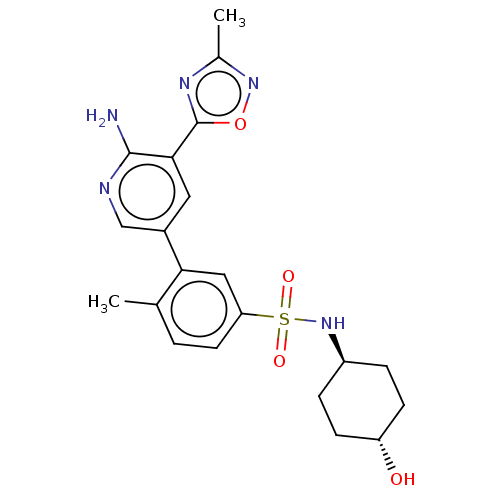 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Binding Assays are based on the binding and displacement of an Alexa Fluor 647-labeled, ATP-competitive kinase inhibitors to the kinase of interest. ... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description The TR-FRET Adapta Universal Kinase Assay Kit was purchased from Invitrogen Corporation (Carlsbad, Calif., USA) (Cat. No. PV5099). The kit contains t... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description The TR-FRET Adapta Universal Kinase Assay Kit was purchased from Invitrogen Corporation (Carlsbad, Calif., USA) (Cat. No. PV5099). The kit contains t... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM295058 (US10112926, Example 12 | trans 3-[6-Amino-5-(3-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description The luminescence-based ATP detection reagent KinaseGlo was obtained from Promega, (Cat. No. V6714, Lot No. 236161) through Catalys, Wallisellen, Swit... | US Patent US10112926 (2018) BindingDB Entry DOI: 10.7270/Q22R3TQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||