 Found 8 hits for monomerid = 444474
Found 8 hits for monomerid = 444474 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monoacylglycerol lipase ABHD6
(Homo sapiens (Human)) | BDBM444474
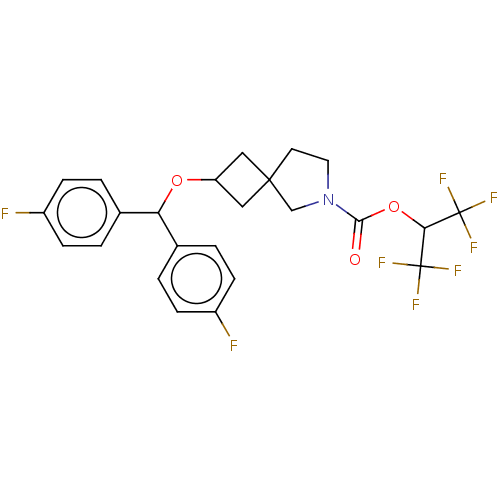
(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
ABHD6: Certain compounds were tested for their ABHD6 and dual ABHD6/MGL inhibitory activity, which is expressed as % of inhibition or IC50 values. Th... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
MGL: Compound inhibition of hMGL activity was assessed by a fluorometric assay recently developed in our laboratory (Makriyannis et al WO Patent Appl... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
Rat/homo FAAH:Procedure was followed as described for hMGL, except that arachidonoyl-methyl coumarin (was used as fluorigenic substrate. Compounds we... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 (aa 30-579)
(Rattus norvegicus (rat)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
hABHD6:Initial Fluorescent Inhibition Assay (3-Point)—In each well of a 96-well plate 8 μL of membrane fraction containing full-length hABHD6 (1... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Monoacylglycerol lipase ABHD6
(Homo sapiens (Human)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
ABHD6: Certain compounds were tested for their ABHD6 and dual ABHD6/MGL inhibitory activity, which is expressed as % of inhibition or IC50 values. Th... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
MGL: Compound inhibition of hMGL activity was assessed by a fluorometric assay recently developed in our laboratory (Makriyannis et al WO Patent Appl... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
Rat/homo FAAH:Procedure was followed as described for hMGL, except that arachidonoyl-methyl coumarin (was used as fluorigenic substrate. Compounds we... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 (aa 30-579)
(Rattus norvegicus (rat)) | BDBM444474

(US10662159, Example 67 | US10662159, Example 68)Show SMILES Fc1ccc(cc1)C(OC1CC2(C1)CCN(C2)C(=O)OC(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 |(-8.79,-.56,;-7.25,-.56,;-6.48,-1.9,;-4.94,-1.9,;-4.17,-.56,;-4.94,.77,;-6.48,.77,;-2.63,-.56,;-1.86,.77,;-.32,.77,;.77,-.32,;1.86,.77,;.77,1.86,;2.76,2.02,;4.23,1.54,;4.23,,;2.76,-.48,;5.47,-.91,;5.31,-2.44,;6.88,-.28,;8.13,-1.18,;9.53,-.56,;10.94,.07,;8.91,.85,;10.16,-1.96,;7.97,-2.72,;7.81,-4.25,;6.43,-2.55,;9.5,-2.88,;-1.86,-1.9,;-.32,-1.9,;.45,-3.23,;-.32,-4.56,;.45,-5.9,;-1.86,-4.56,;-2.63,-3.23,)| Show InChI InChI=1S/C24H21F8NO3/c25-16-5-1-14(2-6-16)19(15-3-7-17(26)8-4-15)35-18-11-22(12-18)9-10-33(13-22)21(34)36-20(23(27,28)29)24(30,31)32/h1-8,18-20H,9-13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MAKSCIENTIFIC, LLC
US Patent
| Assay Description
hABHD6:Initial Fluorescent Inhibition Assay (3-Point)—In each well of a 96-well plate 8 μL of membrane fraction containing full-length hABHD6 (1... |
US Patent US10662159 (2020)
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data







