 Found 6 hits for monomerid = 50079456
Found 6 hits for monomerid = 50079456 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50079456
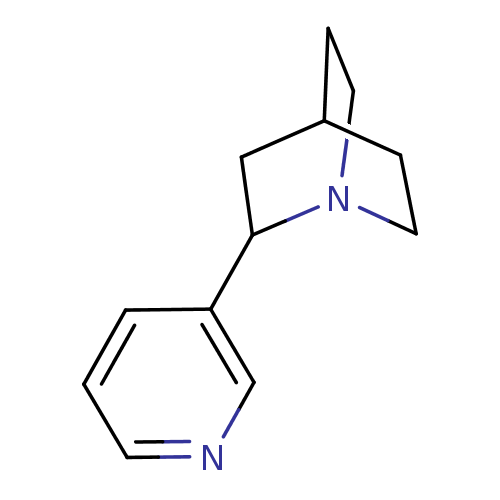
(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-5
(RAT) | BDBM50079456

(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50079456

(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form human alpha7 nAchR expressed in HEK293 cells |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50079456

(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at heteropentameric Nicotinic acetylcholine receptor alpha4-beta2 subtype using [3H]-bungarotoxin as radioligand |
J Med Chem 42: 3066-74 (1999)
Article DOI: 10.1021/jm990093z
BindingDB Entry DOI: 10.7270/Q2445KNP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50079456

(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotine human alpha4beta2 nAChR in SH-EP1 cell membranes |
J Med Chem 55: 9793-809 (2012)
Article DOI: 10.1021/jm301048a
BindingDB Entry DOI: 10.7270/Q2J67J2T |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50079456

(2-Pyridin-3-yl-1-aza-bicyclo[2.2.2]octane | CHEMBL...)Show SMILES C1CN2CCC1CC2c1cccnc1 |(15.84,-6.24,;14.49,-7,;16.04,-8.15,;16.81,-6.8,;16.04,-5.47,;14.49,-5.47,;13.72,-6.8,;14.49,-8.15,;13.17,-8.93,;11.82,-8.15,;10.5,-8.95,;10.5,-10.47,;11.82,-11.27,;13.17,-10.47,)| Show InChI InChI=1S/C12H16N2/c1-2-11(9-13-5-1)12-8-10-3-6-14(12)7-4-10/h1-2,5,9-10,12H,3-4,6-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data