

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50118223 AR-C67085::Adenosine triphosphate derivative::CHEMBL336292::CHEMBL369928
SMILES: CCCSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]3O)c2n1
InChI Key: InChIKey=ZLIAJZQKKBOFJR-WOUKDFQISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purinergic receptor P2Y12 (Homo sapiens (Human)) | BDBM50118223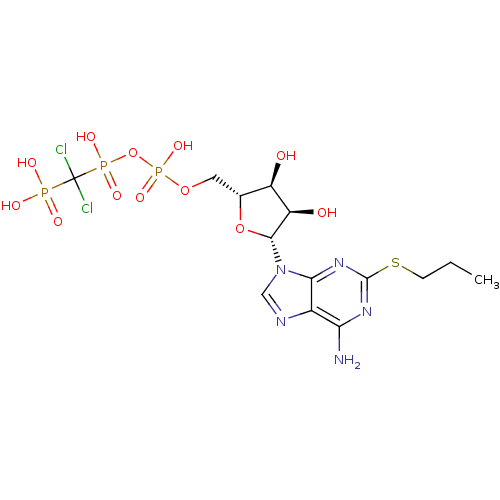 (AR-C67085 | Adenosine triphosphate derivative | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.57 | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Binding affinity to P2Y12 receptor expressed in human platelets from 0.047 to 50 nM | Bioorg Med Chem Lett 15: 5450-2 (2005) Article DOI: 10.1016/j.bmcl.2005.08.104 BindingDB Entry DOI: 10.7270/Q2668CR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y12 (Homo sapiens (Human)) | BDBM50118223 (AR-C67085 | Adenosine triphosphate derivative | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by turbidimetric method | Bioorg Med Chem Lett 26: 2739-2754 (2016) Article DOI: 10.1016/j.bmcl.2016.04.030 BindingDB Entry DOI: 10.7270/Q2FF3V86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50118223 (AR-C67085 | Adenosine triphosphate derivative | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... | Drug Metab Dispos 40: 2332-41 (2012) Article DOI: 10.1124/dmd.112.047068 BindingDB Entry DOI: 10.7270/Q2ZP488M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y12 (Homo sapiens (Human)) | BDBM50118223 (AR-C67085 | Adenosine triphosphate derivative | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against platelet P2Y purinoceptor 12 (P2Y12) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y11 (Homo sapiens (Human)) | BDBM50118223 (AR-C67085 | Adenosine triphosphate derivative | CH...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||