

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50154408 (Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but-2-enoic acid::CHEMBL1673089::CHEMBL186820
SMILES: OC(=O)C(=O)CC(=O)c1cn(Cc2ccccc2)c2ccccc12
InChI Key: InChIKey=KXWMICWZAOIFIN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50154408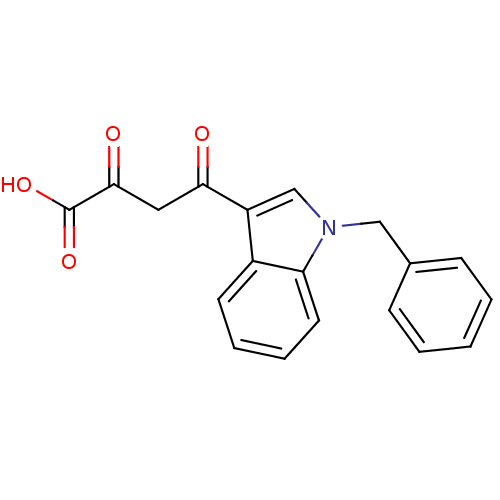 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Inhibition of human CA2 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique | Bioorg Med Chem Lett 22: 5801-6 (2012) Article DOI: 10.1016/j.bmcl.2012.07.094 BindingDB Entry DOI: 10.7270/Q2319X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Inhibition of human CA1 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique | Bioorg Med Chem Lett 22: 5801-6 (2012) Article DOI: 10.1016/j.bmcl.2012.07.094 BindingDB Entry DOI: 10.7270/Q2319X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Inhibition of human CA12 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique | Bioorg Med Chem Lett 22: 5801-6 (2012) Article DOI: 10.1016/j.bmcl.2012.07.094 BindingDB Entry DOI: 10.7270/Q2319X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Inhibition of human CA9 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow technique | Bioorg Med Chem Lett 22: 5801-6 (2012) Article DOI: 10.1016/j.bmcl.2012.07.094 BindingDB Entry DOI: 10.7270/Q2319X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Messina Curated by ChEMBL | Assay Description In vitro concentration required to inhibit the overall HIV-1 integrase strand transfer | J Med Chem 48: 7084-8 (2005) Article DOI: 10.1021/jm050549e BindingDB Entry DOI: 10.7270/Q27W6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Messina Curated by ChEMBL | Assay Description In vitro concentration required to inhibit the HIV-1 integrase strand transfer | J Med Chem 48: 7084-8 (2005) Article DOI: 10.1021/jm050549e BindingDB Entry DOI: 10.7270/Q27W6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 integrase mediated 3' processing reaction | J Med Chem 47: 5298-310 (2004) Article DOI: 10.1021/jm049944f BindingDB Entry DOI: 10.7270/Q2KS6R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 integrase mediated strand transfer reaction | J Med Chem 47: 5298-310 (2004) Article DOI: 10.1021/jm049944f BindingDB Entry DOI: 10.7270/Q2KS6R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50154408 ((Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Messina Curated by ChEMBL | Assay Description In vitro concentration required to inhibit the HIV-1 integrase 3' strand transfer | J Med Chem 48: 7084-8 (2005) Article DOI: 10.1021/jm050549e BindingDB Entry DOI: 10.7270/Q27W6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||