

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50173313 CHEMBL1738878
SMILES: CCCn1c(CNc2cccc(c2)C(=O)NCc2c(F)cccc2F)nnc1-c1ccncn1
InChI Key: InChIKey=VWBSMGFTNCQOMB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50173313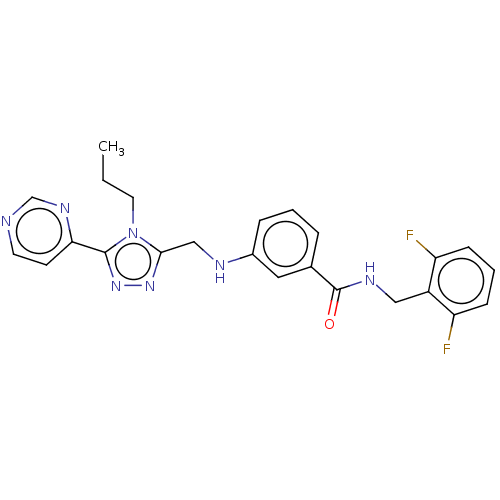 (CHEMBL1738878) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK5 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human PKCalpha active using MBP as substrate after 60 mins in presence of [gamma-32]ATP by scintillation counting | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Bos taurus) | BDBM50173313 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK2 S670A mutant after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase (Bos taurus) | BDBM50173313 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| G protein-coupled receptor kinase 5 (Bos taurus) | BDBM50173313 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards norepinephrine transporter determined using [3H]nisoxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50173313 (CHEMBL1738878) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged ROCK2 catalytic domain (1 to 553 residues) expressed in baculovirus expression system using STK... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||