

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50185446 (E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one::CHEMBL378104::cardamomin::cardamonin
SMILES: COc1cc(O)cc(O)c1C(=O)\C=C\c1ccccc1
InChI Key: InChIKey=NYSZJNUIVUBQMM-BQYQJAHWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dengue virus type 2 NS3 protein (Dengue virus 2) | BDBM50185446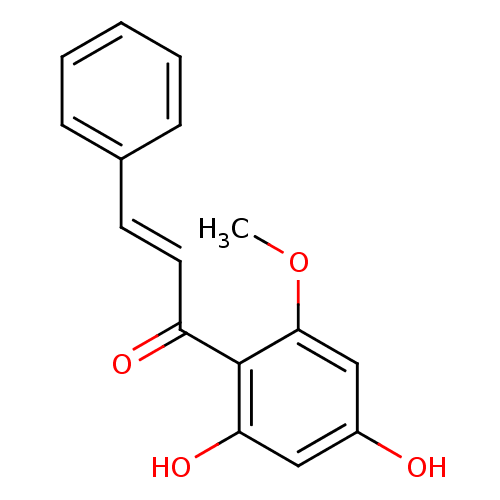 ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunway University College Curated by ChEMBL | Assay Description Inhibition of dengue2 virus NS3 protease | Bioorg Med Chem Lett 16: 3337-40 (2006) Article DOI: 10.1016/j.bmcl.2005.12.075 BindingDB Entry DOI: 10.7270/Q2FN16ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50185446 ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Adam Mickiewicz University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Eur J Med Chem 42: 125-37 (2007) Article DOI: 10.1016/j.ejmech.2006.09.019 BindingDB Entry DOI: 10.7270/Q2CF9SW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated potassium channel (Homo sapiens (Human)) | BDBM50185446 ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human voltage-gated potassium channel Kv1.3 expressed in CGE22 cells by patch clamp assay | Bioorg Med Chem Lett 20: 6983-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.132 BindingDB Entry DOI: 10.7270/Q2N58MMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated potassium channel (Homo sapiens (Human)) | BDBM50185446 ((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human voltage-gated potassium channel Kv1.3 expressed in L929 cells by whole-cell patch clamp assay | Bioorg Med Chem Lett 20: 6983-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.132 BindingDB Entry DOI: 10.7270/Q2N58MMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||