

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50253810 CHEMBL459505::N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)benzo[d]thiazol-2-amine::R-115866::R115866::US9963439, Talarozole
SMILES: CCC(CC)C(c1ccc(Nc2nc3ccccc3s2)cc1)n1cncn1
InChI Key: InChIKey=SNFYYXUGUBUECJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome CYP26A1 (Homo sapiens (Human)) | BDBM50253810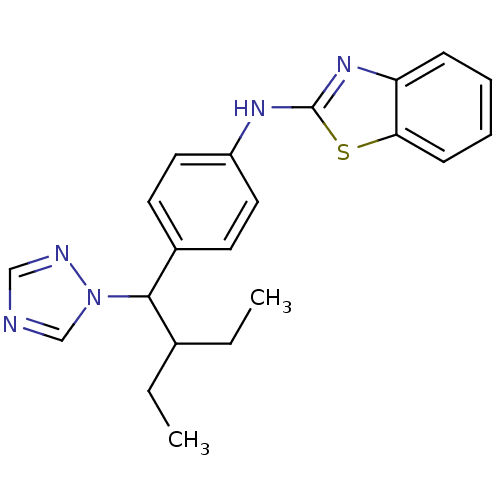 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Cardiff University | Assay Description The imidazole derivatives were evaluated for their retinoic acid metabolism inhibitory activity using a MCF-7 cell assay, using radiolabelled all-tra... | J Enzyme Inhib Med Chem 24: 487-98 (2009) Article DOI: 10.1080/14756360802218334 BindingDB Entry DOI: 10.7270/Q2CR5RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome CYP26A1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26A1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of microsomal fraction of human CYP26B1 expressed in Sf9 cells using 9-cis-RA as substrate preincubated for 5 mins followed by NADPH addit... | J Med Chem 59: 2579-95 (2016) BindingDB Entry DOI: 10.7270/Q2J38VG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26B1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome CYP26A1 (Homo sapiens (Human)) | BDBM50253810 (CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||