

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50293496 2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamido)methyl)phenoxy)acetic acid::CHEMBL563646
SMILES: CC(C)(C)c1ccc(CN(Cc2cccc(OCC(O)=O)c2)S(=O)(=O)c2cccnc2)cc1
InChI Key: InChIKey=WOHRHWDYFNWPNG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50293496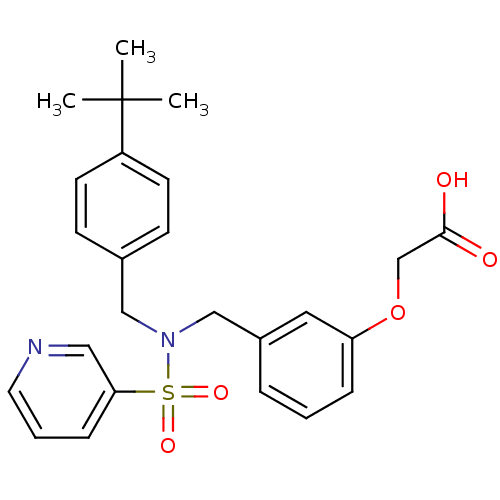 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to EP2 receptor (unknown origin) by competitive binding assay | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP1 subtype (EP1) (Homo sapiens (Human)) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | >2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to EP1 receptor (unknown origin) | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor (Homo sapiens (Human)) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | >2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to EP3 receptor (unknown origin) | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to EP4 receptor (unknown origin) | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to prostanoid IP receptor (unknown origin) | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP4 receptor (Rattus norvegicus) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 2075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.059 BindingDB Entry DOI: 10.7270/Q2PR7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP2 receptor (Rattus norvegicus) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat EP2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 2075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.059 BindingDB Entry DOI: 10.7270/Q2PR7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP2 receptor (Rattus norvegicus) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against rat EP2 receptor expressed in HEK293 cells assessed as stimulation of cAMP release | Bioorg Med Chem Lett 19: 2075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.059 BindingDB Entry DOI: 10.7270/Q2PR7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50293496 (2-(3-((N-(4-tert-butylbenzyl)pyridine-3-sulfonamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at EP2 receptor (unknown origin) by functional assay | J Med Chem 57: 4454-65 (2014) Article DOI: 10.1021/jm401431x BindingDB Entry DOI: 10.7270/Q2CR5VXZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||