

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM7960 1H-1,3-benzodiazol-2-amine::2-Aminobenzimidazole::CHEMBL305513::CRA Fragment 9::Imidazole C-2 deriv. 3::JMC524454 Compound 5
SMILES: Nc1nc2ccccc2[nH]1
InChI Key: InChIKey=JWYUFVNJZUSCSM-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer. 8 PDB IDs contain this monomer as substructures. 15 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM7960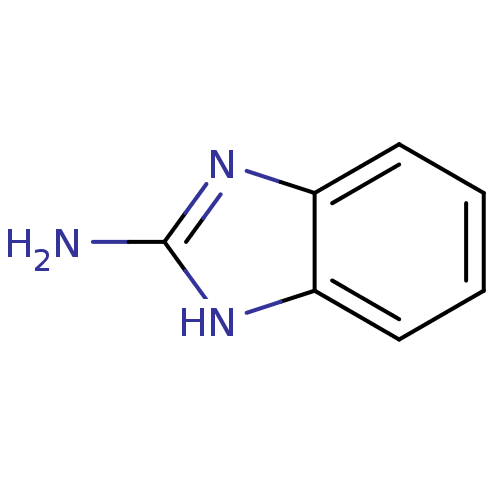 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.10E+5 | -5.34 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | MMDB Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 2.88E+5 | -4.79 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Probiodrug AG | Assay Description QC activity was evaluated fluorometrically using Gln-AMC as substrate, and pyroglutamyl peptidase as the auxiliary enzyme. After conversion of Gln-AM... | J Biol Chem 278: 49773-9 (2003) Article DOI: 10.1074/jbc.M309077200 BindingDB Entry DOI: 10.7270/Q2513WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Binding affinity to recombinant His-tagged MTH1 (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as dissociation constant by isother... | Eur J Med Chem 175: 107-113 (2019) Article DOI: 10.1016/j.ejmech.2019.04.037 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Rattus norvegicus (Rat)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured aft... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human VAP-1 expressed in CHO cells using [14C]-benzylamine as substrate preincubated for 30 mins prior to substrate addition measured a... | Bioorg Med Chem 21: 3873-81 (2013) Article DOI: 10.1016/j.bmc.2013.04.011 BindingDB Entry DOI: 10.7270/Q22B90DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged MTH1 (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP as substrate incubated for 15 min... | Eur J Med Chem 175: 107-113 (2019) Article DOI: 10.1016/j.ejmech.2019.04.037 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Urokinase-type plasminogen activator | J Med Chem 43: 3862-6 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||