

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM81552 Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1::Scaffold, B54
SMILES: CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)N3CCC(O)CC3)nc12
InChI Key: InChIKey=DFQAJLQXPSPNJE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM81552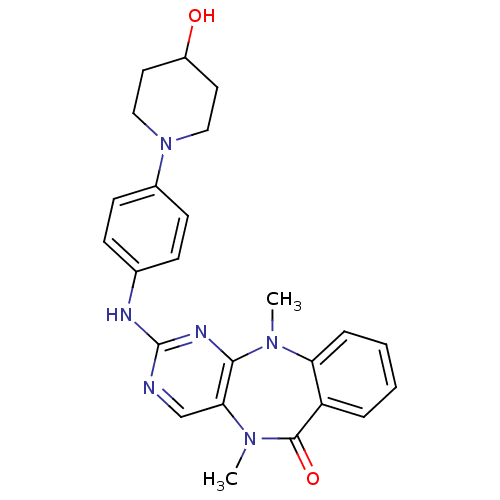 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Aurora-C (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro biochemical assays were performed in parallel to determine the most potent tool compound. | Chem Biol 18: 868-79 (2011) Article DOI: 10.1016/j.chembiol.2011.05.010 BindingDB Entry DOI: 10.7270/Q2HD7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase. | ACS Chem Biol 7: 185-96 (2012) Article DOI: 10.1021/cb200305u BindingDB Entry DOI: 10.7270/Q2PV6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Aurora-C (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase. | ACS Chem Biol 7: 185-96 (2012) Article DOI: 10.1021/cb200305u BindingDB Entry DOI: 10.7270/Q2PV6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM81552 (Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description In vitro kinase assay of Aurora A, B, and C using Z-LYTE technology (Invitrogen) and ATP at Km apparent for each kinase. | ACS Chem Biol 7: 185-96 (2012) Article DOI: 10.1021/cb200305u BindingDB Entry DOI: 10.7270/Q2PV6HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||