

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 200.2 BDBM107306 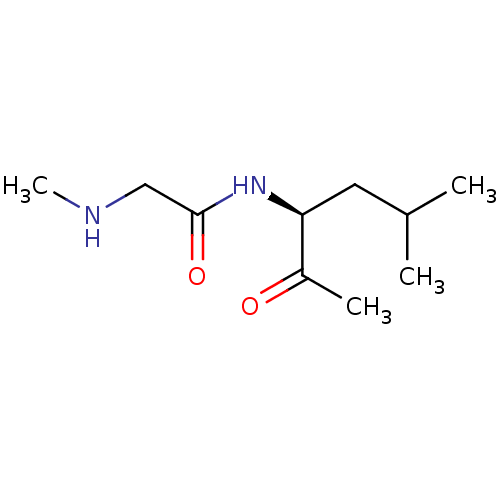 | Wt: 183.2 BDBM107307 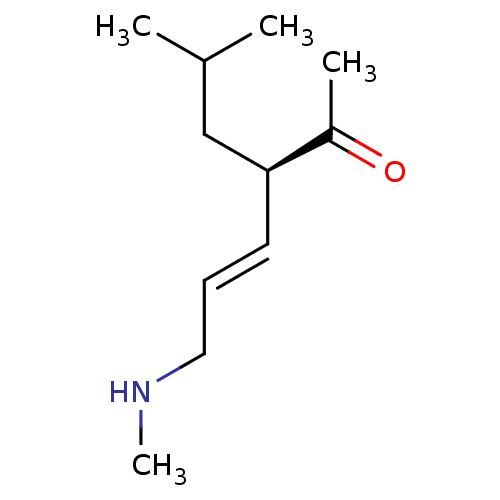 | Wt: 185.3 BDBM107308 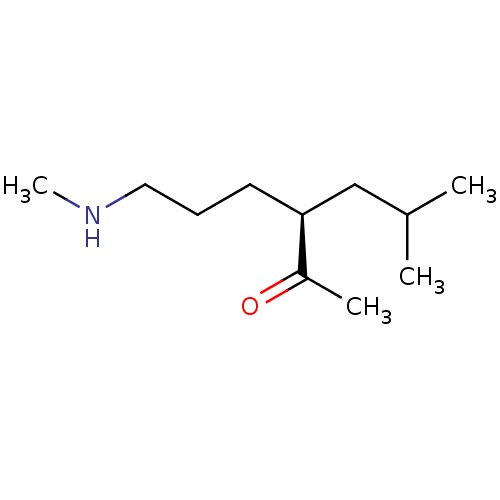 | Wt: 183.2 BDBM107309 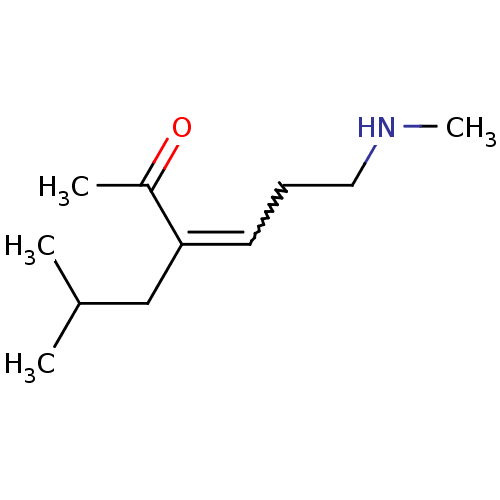 | Wt: 201.3 BDBM107312 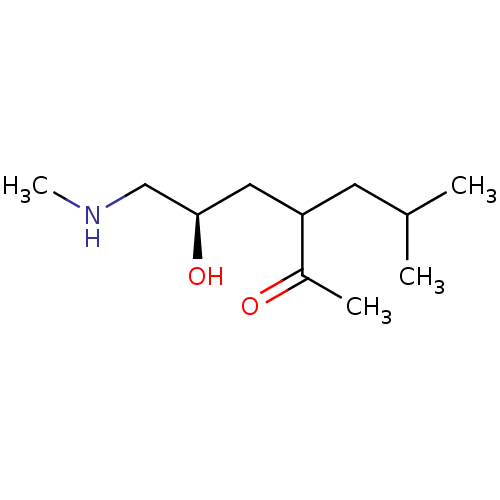 |
| Wt: 201.3 BDBM107314 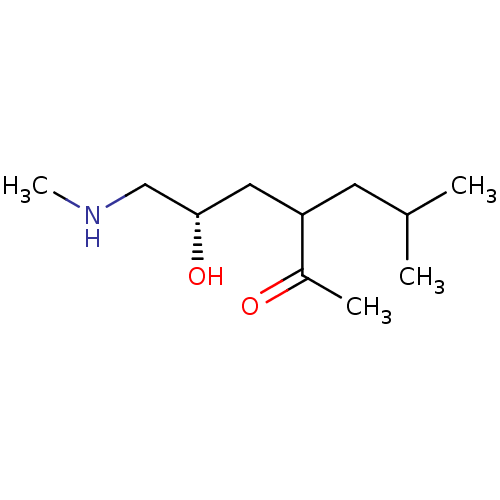 | Wt: 200.2 BDBM107316  | Wt: 412.6 BDBM50203812 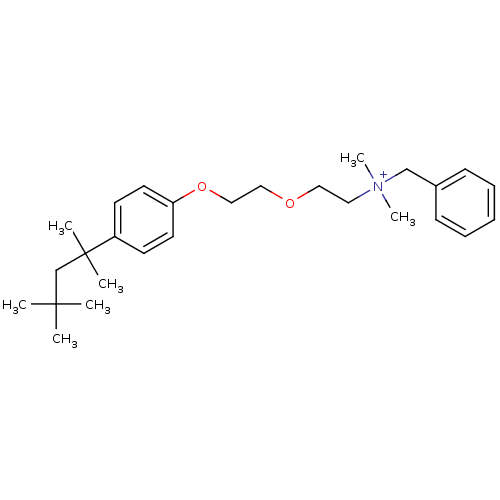 Purchase Purchase | Wt: 412.4 BDBM50320797 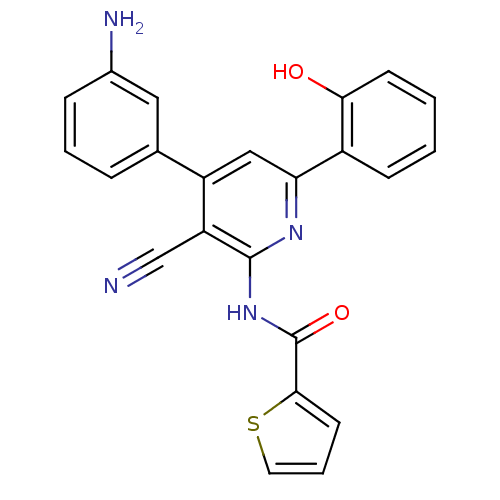 | Wt: 426.4 BDBM50320798 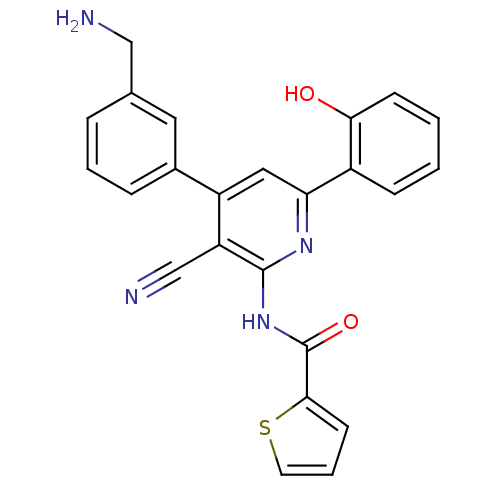 |
| Wt: 426.4 BDBM50320806 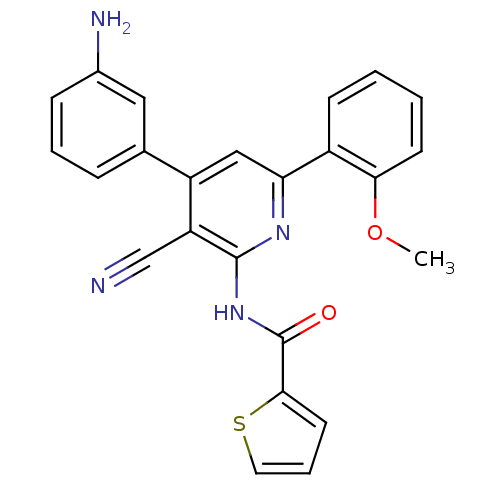 | Wt: 199.2 BDBM107310 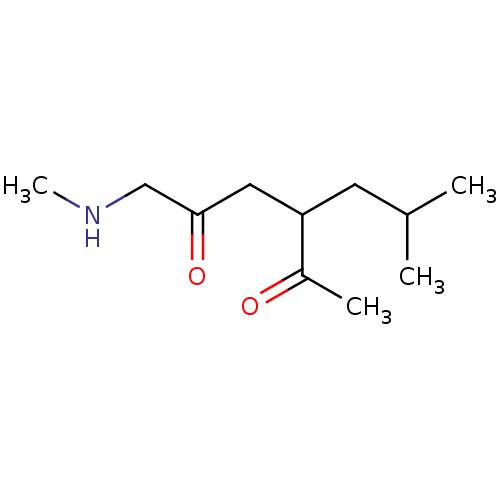 | Wt: 199.2 BDBM47092 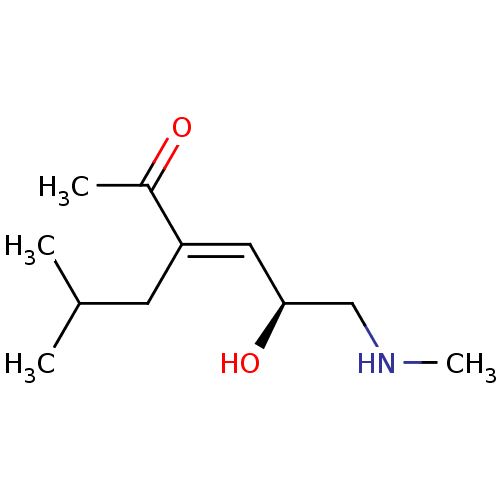 | Wt: 267.5 BDBM50191288 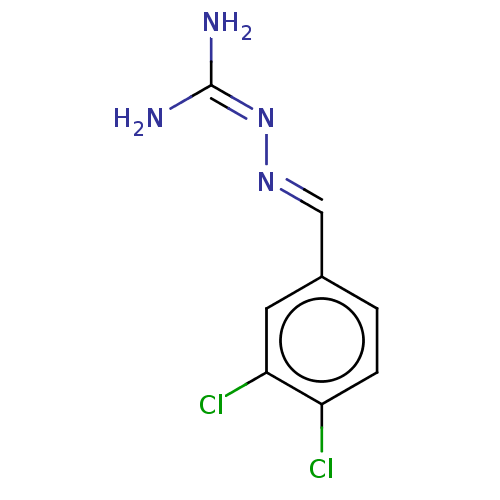 | Wt: 242.1 BDBM50565194  |
| Displayed 1 to 15 (of 316 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50565194 (CHEMBL4793841) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-FFRF-NH2 from human NPFFR1 expressed in CHO cell membrane by Topcount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 2 (Homo sapiens (Human)) | BDBM50565194 (CHEMBL4793841) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-FFRF-NH2 from human NPFFR2 expressed in CHO cell membrane by Topcount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center Curated by ChEMBL | Assay Description Displacement of [125]metastin from metastin receptor | J Med Chem 50: 462-71 (2007) Article DOI: 10.1021/jm0609824 BindingDB Entry DOI: 10.7270/Q2HT2P0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50191288 (CHEMBL3982697) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Kp-10 from human KISS1R expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM50565194 (CHEMBL4793841) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-PrRP-20 from human PrRP receptor expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM50191288 (CHEMBL3982697) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-PrRP-20 from human PrRP receptor expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50191288 (CHEMBL3982697) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-43RFa from human QRFP receptor expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyroglutamylated RF-amide peptide receptor (Homo sapiens (Human)) | BDBM50565194 (CHEMBL4793841) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-43RFa from human QRFP receptor expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50565194 (CHEMBL4793841) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Kp-10 from human KISS1R expressed in CHO cell membrane by TopCount scintillation counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107307 (US8592379, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107314 (US8592379, 36 | US8592379, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107306 (US8592379, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107308 (US8592379, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107309 (US8592379, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107312 (US8592379, 24a/24b | US8592379, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107314 (US8592379, 36 | US8592379, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107310 (US8592379, 22 | US8592379, 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107312 (US8592379, 24a/24b | US8592379, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM47092 (US8592379, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50320798 (CHEMBL1164673 | N-{4-[3-(Aminomethyl)phenyl]-3-cya...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Displacement of [125I]metastin(40-54) from human GPR54 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 18: 3841-59 (2010) Article DOI: 10.1016/j.bmc.2010.04.036 BindingDB Entry DOI: 10.7270/Q22807S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50320797 (CHEMBL1164603 | N-[4-(3-Aminophenyl)-3-cyano-6-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Displacement of [125I]metastin(40-54) from human GPR54 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 18: 3841-59 (2010) Article DOI: 10.1016/j.bmc.2010.04.036 BindingDB Entry DOI: 10.7270/Q22807S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM107316 (US8592379, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University; Takeda Pharmaceutical Company Limited US Patent | Assay Description Binding inhibition assay using human GPR54. | US Patent US8592379 (2013) BindingDB Entry DOI: 10.7270/Q2N0156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-conjugating enzyme E2 N (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2X34VX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 60 kDa heat shock protein, mitochondrial (Homo sapiens) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal octa-His-tagged HSP60 expressed in Escherichia coli Rosetta(DE3) pLysS/human HSP10 expressed in Escherichia coli Roset... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic protease-activating factor 1 (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2JS9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coliDH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed a... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50320806 (CHEMBL1163805 | N-[4-(3-Aminophenyl)-3-cyano-6-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Displacement of [125I]metastin(40-54) from human GPR54 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 18: 3841-59 (2010) Article DOI: 10.1016/j.bmc.2010.04.036 BindingDB Entry DOI: 10.7270/Q22807S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25M647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic protease-activating factor 1 (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2F18X8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chaperonin GroEL (Escherichia coli) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Escherichia coli GroEL expressed in Escherichia coliDH5alpha incubated for 60 mins using ATP by spectrometric analys... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Iron-starvation protein PigA (Pseudomonas aeruginosa) | BDBM50191288 (CHEMBL3982697) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to Pseudomonas aeruginosa HemO by intrinsic fluorescence quenching method | J Med Chem 59: 6929-42 (2016) Article DOI: 10.1021/acs.jmedchem.6b00757 BindingDB Entry DOI: 10.7270/Q26W9D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >8.92E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute, TS... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2QC0233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 2 (Homo sapiens (Human)) | BDBM50191288 (CHEMBL3982697) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at NPFFR2 (unknown origin) expressed in mouse NIH3T3 cells by receptor selection and amplification technology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50203812 (CHEMBL221753 | benzethonium hydrochloride | cid_84...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >9.25E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute, TS... | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2KK99CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||