 Found 1784 hits with Last Name = 'gros' and Initial = 'a'
Found 1784 hits with Last Name = 'gros' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510
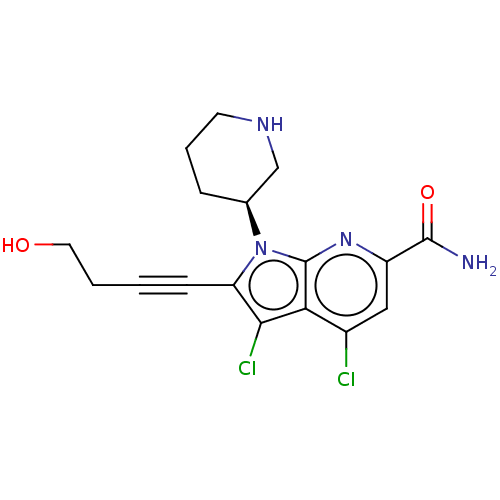
(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108018
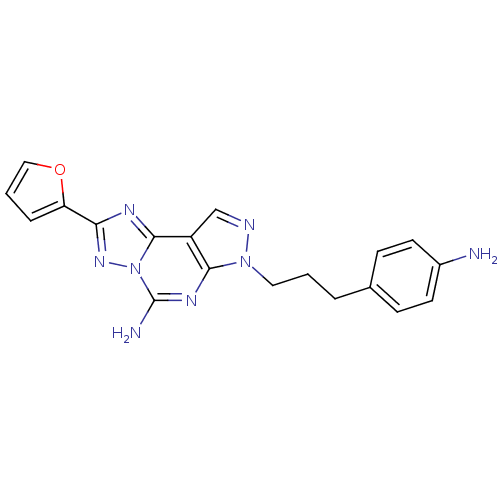
(7-(3-(4-aminophenyl)propyl)-2-(furan-2-yl)-7H-pyra...)Show SMILES Nc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C19H18N8O/c20-13-7-5-12(6-8-13)3-1-9-26-17-14(11-22-26)18-23-16(15-4-2-10-28-15)25-27(18)19(21)24-17/h2,4-8,10-11H,1,3,9,20H2,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against WT human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against Q89D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517
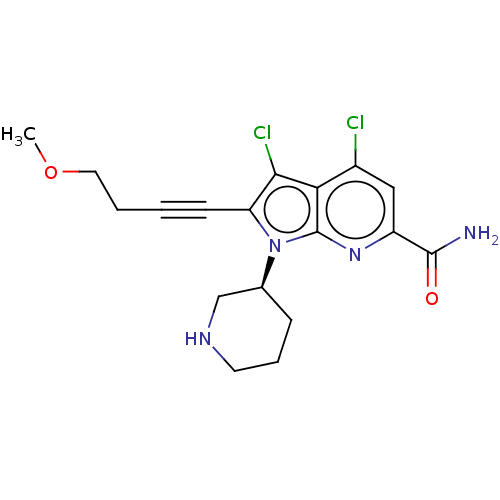
(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against H278D human adenosine A2A receptor stably transfected in CHO cells using [3H]- ZM-241385 as radioligand. |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against H278E human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against T88E human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against WT human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518507

(CHEMBL4583118)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CNCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H21Cl2N5O2/c20-13-8-14(18(22)27)25-19-16(13)17(21)15(4-3-12-10-24-6-7-28-12)26(19)11-2-1-5-23-9-11/h8,11-12,23-24H,1-2,5-7,9-10H2,(H2,22,27)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against T88D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against S277E human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518514
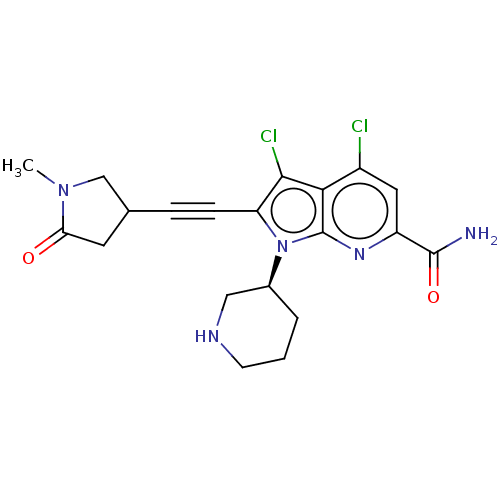
(CHEMBL4563802)Show SMILES CN1CC(CC1=O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H21Cl2N5O2/c1-26-10-11(7-16(26)28)4-5-15-18(22)17-13(21)8-14(19(23)29)25-20(17)27(15)12-3-2-6-24-9-12/h8,11-12,24H,2-3,6-7,9-10H2,1H3,(H2,23,29)/t11?,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518
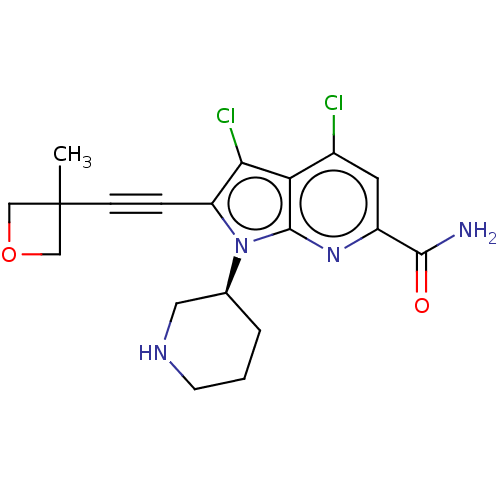
(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518505
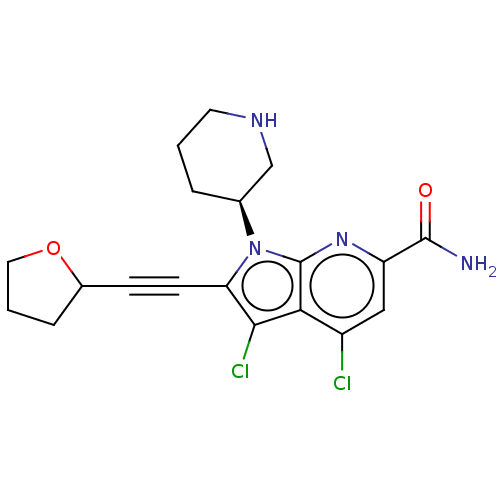
(CHEMBL4518492)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O2/c20-13-9-14(18(22)26)24-19-16(13)17(21)15(6-5-12-4-2-8-27-12)25(19)11-3-1-7-23-10-11/h9,11-12,23H,1-4,7-8,10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against Q89D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50124895
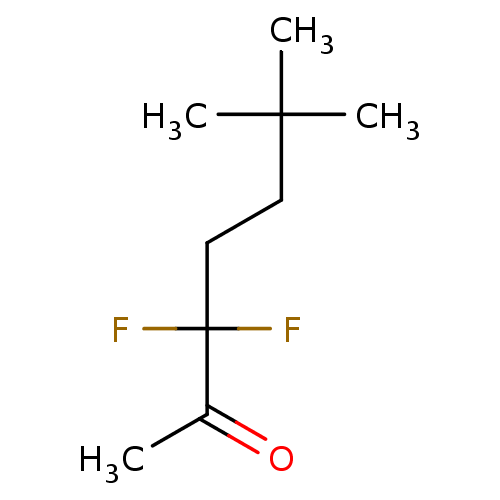
(CHEMBL3623568)Show InChI InChI=1S/C9H16F2O/c1-7(12)9(10,11)6-5-8(2,3)4/h5-6H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem Lett 25: 4405-11 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.019
BindingDB Entry DOI: 10.7270/Q2Z60QV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518522

(CHEMBL4466080)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C16H16Cl2N4O2/c17-10-7-11(15(19)24)21-16-13(10)14(18)12(4-2-6-23)22(16)9-3-1-5-20-8-9/h7,9,20,23H,1,3,5-6,8H2,(H2,19,24)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50106539

((2S,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES NCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H17N7O4/c13-1-2-15-11(22)8-6(20)7(21)12(23-8)19-4-18-5-9(14)16-3-17-10(5)19/h3-4,6-8,12,20-21H,1-2,13H2,(H,15,22)(H2,14,16,17)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against Q89D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50106539

((2S,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES NCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H17N7O4/c13-1-2-15-11(22)8-6(20)7(21)12(23-8)19-4-18-5-9(14)16-3-17-10(5)19/h3-4,6-8,12,20-21H,1-2,13H2,(H,15,22)(H2,14,16,17)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against T88D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50106535

((2R,3R,4S,5S)-4-Amino-5-hydroxymethyl-2-[6-(3-iodo...)Show SMILES N[C@@H]1[C@@H](CO)O[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C17H19IN6O3/c18-10-3-1-2-9(4-10)5-20-15-13-16(22-7-21-15)24(8-23-13)17-14(26)12(19)11(6-25)27-17/h1-4,7-8,11-12,14,17,25-26H,5-6,19H2,(H,20,21,22)/t11-,12-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against Q89D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50134814
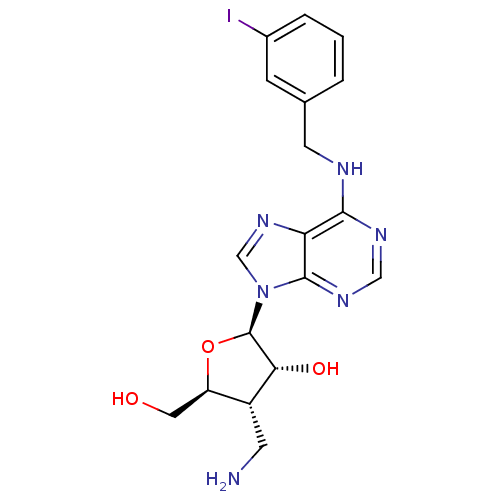
((2R,3R,4S,5S)-2-(6-(3-iodobenzylamino)-9H-purin-9-...)Show SMILES NC[C@@H]1[C@@H](CO)O[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H21IN6O3/c19-11-3-1-2-10(4-11)6-21-16-14-17(23-8-22-16)25(9-24-14)18-15(27)12(5-20)13(7-26)28-18/h1-4,8-9,12-13,15,18,26-27H,5-7,20H2,(H,21,22,23)/t12-,13-,15-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against T88D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50428509
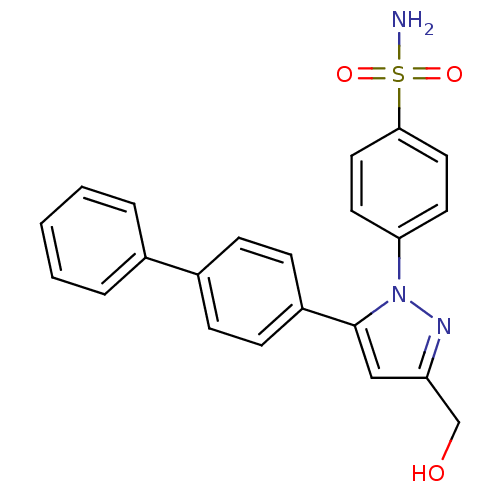
(CHEMBL2331608)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O3S/c23-29(27,28)21-12-10-20(11-13-21)25-22(14-19(15-26)24-25)18-8-6-17(7-9-18)16-4-2-1-3-5-16/h1-14,26H,15H2,(H2,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50428503

(CHEMBL2336615)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1CO)-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H17N3O3S/c21-27(25,26)19-9-7-17(8-10-19)23-18(13-24)12-20(22-23)16-6-5-14-3-1-2-4-15(14)11-16/h1-12,24H,13H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50428512

(CHEMBL2336626)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H17N3O3S/c21-27(25,26)19-9-7-18(8-10-19)23-20(12-17(13-24)22-23)16-6-5-14-3-1-2-4-15(14)11-16/h1-12,24H,13H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428520
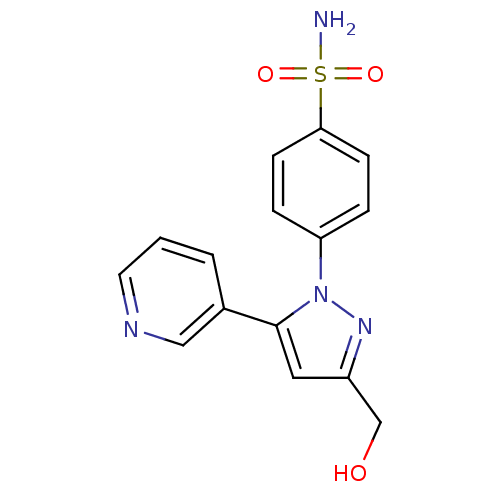
(CHEMBL2336632)Show InChI InChI=1S/C15H14N4O3S/c16-23(21,22)14-5-3-13(4-6-14)19-15(8-12(10-20)18-19)11-2-1-7-17-9-11/h1-9,20H,10H2,(H2,16,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428518

(CHEMBL2336620)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc(Br)cc1 Show InChI InChI=1S/C16H14BrN3O3S/c17-12-3-1-11(2-4-12)16-9-13(10-21)19-20(16)14-5-7-15(8-6-14)24(18,22)23/h1-9,21H,10H2,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428512

(CHEMBL2336626)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H17N3O3S/c21-27(25,26)19-9-7-18(8-10-19)23-20(12-17(13-24)22-23)16-6-5-14-3-1-2-4-15(14)11-16/h1-12,24H,13H2,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50106535

((2R,3R,4S,5S)-4-Amino-5-hydroxymethyl-2-[6-(3-iodo...)Show SMILES N[C@@H]1[C@@H](CO)O[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C17H19IN6O3/c18-10-3-1-2-9(4-10)5-20-15-13-16(22-7-21-15)24(8-23-13)17-14(26)12(19)11(6-25)27-17/h1-4,7-8,11-12,14,17,25-26H,5-6,19H2,(H,20,21,22)/t11-,12-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against T88D human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428511

(CHEMBL2336627)Show SMILES COc1ccc2cc(ccc2c1)-c1cc(CO)nn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H19N3O4S/c1-28-19-7-4-14-10-16(3-2-15(14)11-19)21-12-17(13-25)23-24(21)18-5-8-20(9-6-18)29(22,26)27/h2-12,25H,13H2,1H3,(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428501

(CHEMBL2336617)Show SMILES NS(=O)(=O)c1cccc(c1)-n1nc(CO)cc1-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H17N3O3S/c21-27(25,26)19-7-3-6-18(12-19)23-20(11-17(13-24)22-23)16-9-8-14-4-1-2-5-15(14)10-16/h1-12,24H,13H2,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against H278D human adenosine A2A receptor stably transfected in CHO cells using [3H]- ZM-241385 as radioligand. |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428516

(CHEMBL2336622)Show SMILES COc1ccc(cc1)-c1cc(CO)nn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H17N3O4S/c1-24-15-6-2-12(3-7-15)17-10-13(11-21)19-20(17)14-4-8-16(9-5-14)25(18,22)23/h2-10,21H,11H2,1H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428503

(CHEMBL2336615)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1CO)-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H17N3O3S/c21-27(25,26)19-9-7-17(8-10-19)23-18(13-24)12-20(22-23)16-6-5-14-3-1-2-4-15(14)11-16/h1-12,24H,13H2,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 21.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against WT human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428519

(CHEMBL2336619)Show SMILES Cc1ccc(cc1)-c1cc(CO)nn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H17N3O3S/c1-12-2-4-13(5-3-12)17-10-14(11-21)19-20(17)15-6-8-16(9-7-15)24(18,22)23/h2-10,21H,11H2,1H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428521

(CHEMBL2336631)Show InChI InChI=1S/C17H17N3O3S/c18-24(22,23)16-8-6-15(7-9-16)20-17(12-14(19-20)10-11-21)13-4-2-1-3-5-13/h1-9,12,21H,10-11H2,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 24.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against H278E human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428515

(CHEMBL2336623)Show SMILES COc1cccc(c1)-c1cc(CO)nn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H17N3O4S/c1-24-15-4-2-3-12(9-15)17-10-13(11-21)19-20(17)14-5-7-16(8-6-14)25(18,22)23/h2-10,21H,11H2,1H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428522

(CHEMBL2336633)Show InChI InChI=1S/C16H15N3O3S/c17-23(21,22)15-8-6-14(7-9-15)19-16(10-13(11-20)18-19)12-4-2-1-3-5-12/h1-10,20H,11H2,(H2,17,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428509

(CHEMBL2331608)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O3S/c23-29(27,28)21-12-10-20(11-13-21)25-22(14-19(15-26)24-25)18-8-6-17(7-9-18)16-4-2-1-3-5-16/h1-14,26H,15H2,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428517

(CHEMBL2336621)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(CO)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C17H14N4O3S/c18-10-12-1-3-13(4-2-12)17-9-14(11-22)20-21(17)15-5-7-16(8-6-15)25(19,23)24/h1-9,22H,11H2,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428500

(CHEMBL2336618)Show SMILES COc1ccc2cc(ccc2c1)-c1cc(CO)nn1-c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C21H19N3O4S/c1-28-19-8-7-14-9-16(6-5-15(14)10-19)21-11-17(13-25)23-24(21)18-3-2-4-20(12-18)29(22,26)27/h2-12,25H,13H2,1H3,(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 29.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity against S277E human adenosine A2A receptor expressed in CHO cells using [3H]- ZM-241385 |
J Med Chem 46: 4847-59 (2003)
Article DOI: 10.1021/jm0300431
BindingDB Entry DOI: 10.7270/Q24M9584 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428510
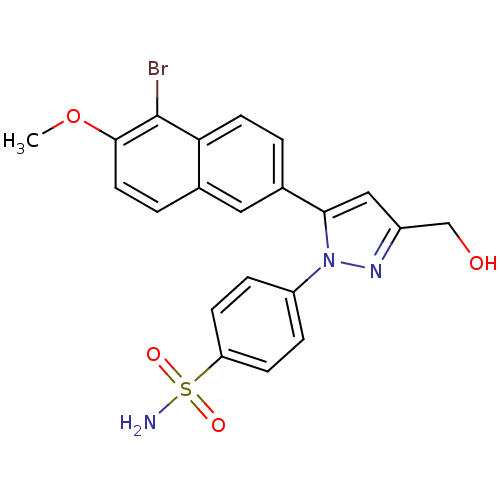
(CHEMBL2336628)Show SMILES COc1ccc2cc(ccc2c1Br)-c1cc(CO)nn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18BrN3O4S/c1-29-20-9-3-13-10-14(2-8-18(13)21(20)22)19-11-15(12-26)24-25(19)16-4-6-17(7-5-16)30(23,27)28/h2-11,26H,12H2,1H3,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50428505

(CHEMBL2336613)Show SMILES COc1ccc2cc(ccc2c1)-c1nn(cc1CO)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H19N3O4S/c1-28-19-7-4-14-10-16(3-2-15(14)11-19)21-17(13-25)12-24(23-21)18-5-8-20(9-6-18)29(22,26)27/h2-12,25H,13H2,1H3,(H2,22,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50428502
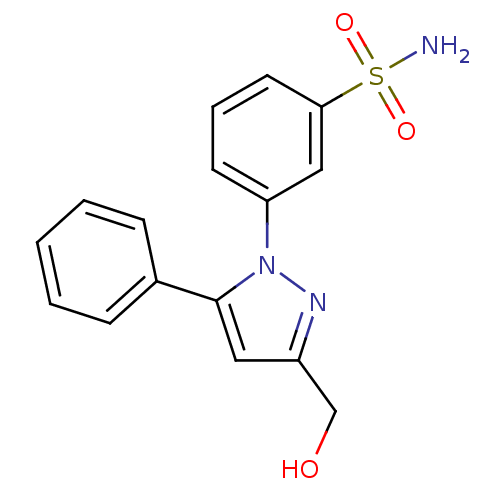
(CHEMBL2336616)Show InChI InChI=1S/C16H15N3O3S/c17-23(21,22)15-8-4-7-14(10-15)19-16(9-13(11-20)18-19)12-5-2-1-3-6-12/h1-10,20H,11H2,(H2,17,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 preincubated for 10 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem 21: 1451-64 (2013)
Article DOI: 10.1016/j.bmc.2012.10.029
BindingDB Entry DOI: 10.7270/Q2ZS2XVC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data