 Found 253 hits with Last Name = 'omura' and Initial = 'a'
Found 253 hits with Last Name = 'omura' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375611

(CHEMBL408120)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N\OCC(=O)NCCCNC(=O)c3ccc(F)c(c3)N=[N+]=[N-])O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C53H81FN6O16/c1-28(2)47(74-45(66)26-41(63)46(52(70)71)34(8)51(68)69)49(72-10)42(64)25-40(62)33(7)39(61)16-12-31(5)48-32(6)19-21-53(76-48)20-18-30(4)43(75-53)17-13-29(3)35(9)59-73-27-44(65)56-22-11-23-57-50(67)36-14-15-37(54)38(24-36)58-60-55/h14-15,24,28-33,39,41-43,47-49,61,63-64H,11-13,16-23,25-27H2,1-10H3,(H,56,65)(H,57,67)(H,68,69)(H,70,71)/b46-34-,59-35+/t29-,30+,31+,32-,33-,39-,41+,42+,43-,47+,48-,49-,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375608

(CHEMBL406220)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C |r| Show InChI InChI=1S/C41H68O14/c1-21(2)36(53-34(47)20-31(45)35(40(50)51)27(8)39(48)49)38(52-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(55-37)17-15-23(4)33(54-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3,(H,48,49)(H,50,51)/b35-27-/t22-,23+,24+,25-,26-,29-,31+,32+,33-,36+,37-,38-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375616
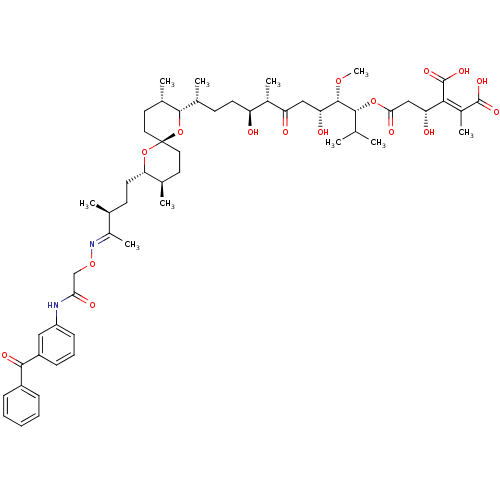
(CHEMBL405248)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N\OCC(=O)Nc3cccc(c3)C(=O)c3ccccc3)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C56H80N2O16/c1-31(2)51(72-48(64)29-44(61)49(55(68)69)37(8)54(66)67)53(70-10)45(62)28-43(60)36(7)42(59)21-19-34(5)52-35(6)24-26-56(74-52)25-23-33(4)46(73-56)22-20-32(3)38(9)58-71-30-47(63)57-41-18-14-17-40(27-41)50(65)39-15-12-11-13-16-39/h11-18,27,31-36,42,44-46,51-53,59,61-62H,19-26,28-30H2,1-10H3,(H,57,63)(H,66,67)(H,68,69)/b49-37-,58-38+/t32-,33+,34+,35-,36-,42-,44+,45+,46-,51+,52-,53-,56+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375613

(CHEMBL261577)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N/OCC(=O)NCCNC(=O)c3ccc(F)c(c3)N=[N+]=[N-])O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C52H79FN6O16/c1-27(2)46(73-44(65)25-40(62)45(51(69)70)33(8)50(67)68)48(71-10)41(63)24-39(61)32(7)38(60)15-11-30(5)47-31(6)18-20-52(75-47)19-17-29(4)42(74-52)16-12-28(3)34(9)58-72-26-43(64)55-21-22-56-49(66)35-13-14-36(53)37(23-35)57-59-54/h13-14,23,27-32,38,40-42,46-48,60,62-63H,11-12,15-22,24-26H2,1-10H3,(H,55,64)(H,56,66)(H,67,68)(H,69,70)/b45-33-,58-34-/t28-,29+,30+,31-,32-,38-,40+,41+,42-,46+,47-,48-,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375612
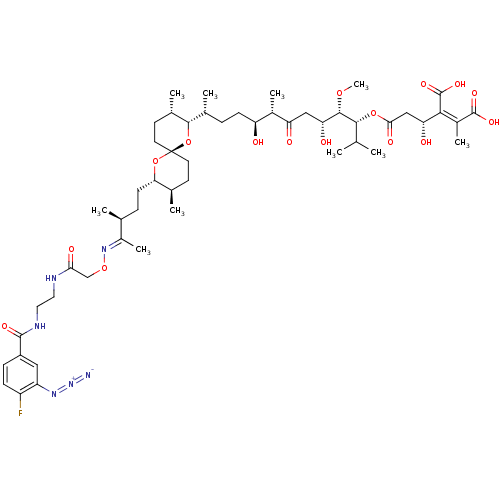
(CHEMBL427915)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N\OCC(=O)NCCNC(=O)c3ccc(F)c(c3)N=[N+]=[N-])O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C52H79FN6O16/c1-27(2)46(73-44(65)25-40(62)45(51(69)70)33(8)50(67)68)48(71-10)41(63)24-39(61)32(7)38(60)15-11-30(5)47-31(6)18-20-52(75-47)19-17-29(4)42(74-52)16-12-28(3)34(9)58-72-26-43(64)55-21-22-56-49(66)35-13-14-36(53)37(23-35)57-59-54/h13-14,23,27-32,38,40-42,46-48,60,62-63H,11-12,15-22,24-26H2,1-10H3,(H,55,64)(H,56,66)(H,67,68)(H,69,70)/b45-33-,58-34+/t28-,29+,30+,31-,32-,38-,40+,41+,42-,46+,47-,48-,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375609
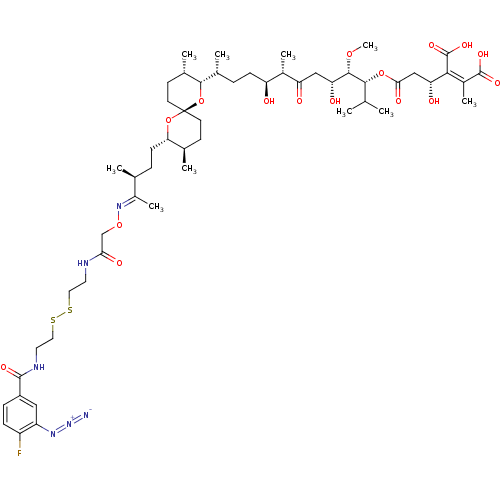
(CHEMBL406283)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N\OCC(=O)NCCSSCCNC(=O)c3ccc(F)c(c3)N=[N+]=[N-])O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C54H83FN6O16S2/c1-29(2)48(75-46(67)27-42(64)47(53(71)72)35(8)52(69)70)50(73-10)43(65)26-41(63)34(7)40(62)15-11-32(5)49-33(6)18-20-54(77-49)19-17-31(4)44(76-54)16-12-30(3)36(9)60-74-28-45(66)57-21-23-78-79-24-22-58-51(68)37-13-14-38(55)39(25-37)59-61-56/h13-14,25,29-34,40,42-44,48-50,62,64-65H,11-12,15-24,26-28H2,1-10H3,(H,57,66)(H,58,68)(H,69,70)(H,71,72)/b47-35-,60-36+/t30-,31+,32+,33-,34-,40-,42+,43+,44-,48+,49-,50-,54+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375614
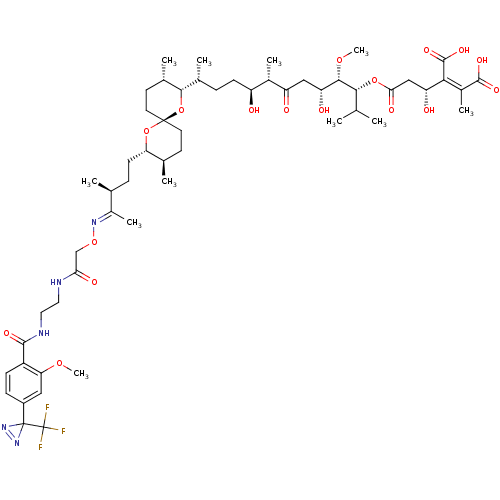
(CHEMBL259235)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N\OCC(=O)NCCNC(=O)c3ccc(cc3OC)C3(N=N3)C(F)(F)F)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C |c:51| Show InChI InChI=1S/C55H82F3N5O17/c1-28(2)47(78-45(69)26-40(66)46(52(73)74)34(8)51(71)72)49(76-11)41(67)25-39(65)33(7)38(64)16-12-31(5)48-32(6)19-21-53(80-48)20-18-30(4)42(79-53)17-13-29(3)35(9)61-77-27-44(68)59-22-23-60-50(70)37-15-14-36(24-43(37)75-10)54(62-63-54)55(56,57)58/h14-15,24,28-33,38,40-42,47-49,64,66-67H,12-13,16-23,25-27H2,1-11H3,(H,59,68)(H,60,70)(H,71,72)(H,73,74)/b46-34-,61-35+/t29-,30+,31+,32-,33-,38-,40+,41+,42-,47+,48-,49-,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375610
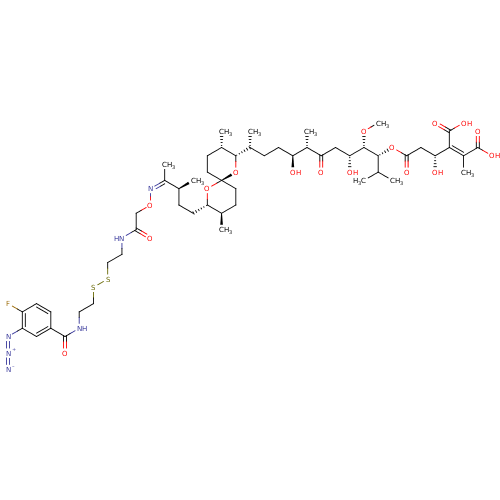
(CHEMBL436904)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N/OCC(=O)NCCSSCCNC(=O)c3ccc(F)c(c3)N=[N+]=[N-])O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C54H83FN6O16S2/c1-29(2)48(75-46(67)27-42(64)47(53(71)72)35(8)52(69)70)50(73-10)43(65)26-41(63)34(7)40(62)15-11-32(5)49-33(6)18-20-54(77-49)19-17-31(4)44(76-54)16-12-30(3)36(9)60-74-28-45(66)57-21-23-78-79-24-22-58-51(68)37-13-14-38(55)39(25-37)59-61-56/h13-14,25,29-34,40,42-44,48-50,62,64-65H,11-12,15-24,26-28H2,1-10H3,(H,57,66)(H,58,68)(H,69,70)(H,71,72)/b47-35-,60-36-/t30-,31+,32+,33-,34-,40-,42+,43+,44-,48+,49-,50-,54+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375617

(CHEMBL261094)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N/OCC(=O)Nc3cccc(c3)C(=O)c3ccccc3)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C Show InChI InChI=1S/C56H80N2O16/c1-31(2)51(72-48(64)29-44(61)49(55(68)69)37(8)54(66)67)53(70-10)45(62)28-43(60)36(7)42(59)21-19-34(5)52-35(6)24-26-56(74-52)25-23-33(4)46(73-56)22-20-32(3)38(9)58-71-30-47(63)57-41-18-14-17-40(27-41)50(65)39-15-12-11-13-16-39/h11-18,27,31-36,42,44-46,51-53,59,61-62H,19-26,28-30H2,1-10H3,(H,57,63)(H,66,67)(H,68,69)/b49-37-,58-38-/t32-,33+,34+,35-,36-,42-,44+,45+,46-,51+,52-,53-,56+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50375615
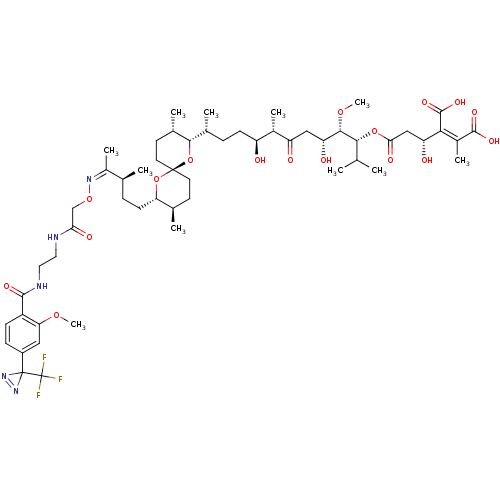
(CHEMBL261583)Show SMILES CO[C@@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(\C)=N/OCC(=O)NCCNC(=O)c3ccc(cc3OC)C3(N=N3)C(F)(F)F)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C(\C(O)=O)=C(/C)C(O)=O)C(C)C |c:51| Show InChI InChI=1S/C55H82F3N5O17/c1-28(2)47(78-45(69)26-40(66)46(52(73)74)34(8)51(71)72)49(76-11)41(67)25-39(65)33(7)38(64)16-12-31(5)48-32(6)19-21-53(80-48)20-18-30(4)42(79-53)17-13-29(3)35(9)61-77-27-44(68)59-22-23-60-50(70)37-15-14-36(24-43(37)75-10)54(62-63-54)55(56,57)58/h14-15,24,28-33,38,40-42,47-49,64,66-67H,12-13,16-23,25-27H2,1-11H3,(H,59,68)(H,60,70)(H,71,72)(H,73,74)/b46-34-,61-35-/t29-,30+,31+,32-,33-,38-,40+,41+,42-,47+,48-,49-,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya University
Curated by ChEMBL
| Assay Description
Inhibition of PP1gamma by firefly bioluminescence assay |
Bioorg Med Chem 16: 1747-55 (2008)
Article DOI: 10.1016/j.bmc.2007.11.034
BindingDB Entry DOI: 10.7270/Q2SJ1MHT |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557765

(CHEMBL4791414)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557761

(CHEMBL4758580)Show SMILES COc1cn2ncc(-c3ccnc(OC(C)C)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557764

(CHEMBL4787891)Show SMILES COc1cn2ncc(-c3ccnc(OC4CCC4)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557766

(CHEMBL4751832)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557760

(CHEMBL4749011)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557742

(CHEMBL4752199) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557753

(CHEMBL4748097) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557756

(CHEMBL4779273) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557755

(CHEMBL4782729) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557744

(CHEMBL4741185) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557763

(CHEMBL4744618)Show SMILES COc1cn2ncc(-c3ccnc(OC4CC4)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557767

(CHEMBL4764221)Show SMILES Cn1cc(cn1)-c1nc2c(cnn2cc1OCCCC(C)(C)O)-c1ccnc(OC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557746
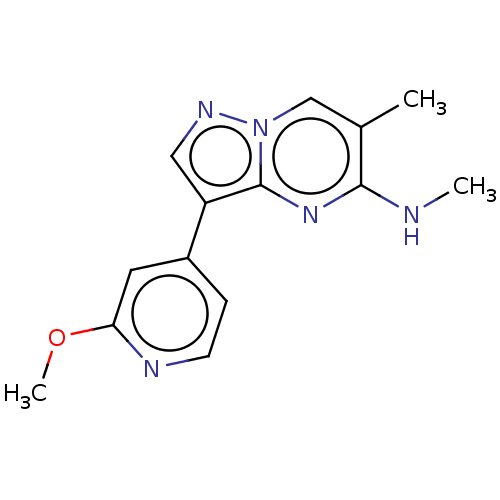
(CHEMBL4779579) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557759

(CHEMBL4763627)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)-c1cc(C)no1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557745

(CHEMBL4741913) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557757

(CHEMBL4792464)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)[C@H]1COC(C)(C)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557754

(CHEMBL4764416) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557758
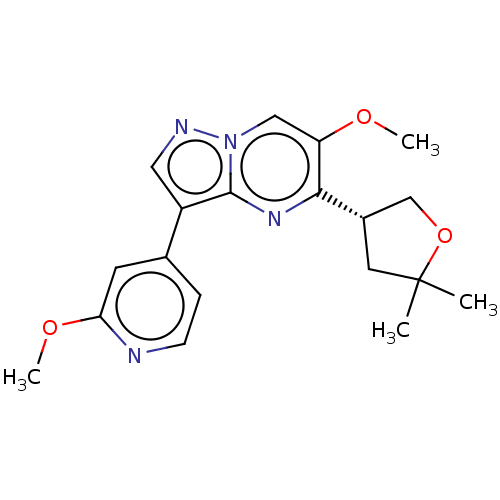
(CHEMBL4749061)Show SMILES COc1cc(ccn1)-c1cnn2cc(OC)c(nc12)[C@@H]1COC(C)(C)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557743
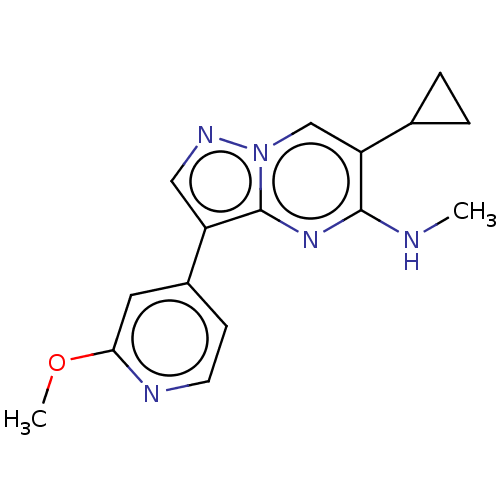
(CHEMBL4744988) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557747

(CHEMBL4795223) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557739

(CHEMBL4798505) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346122

(2-(4-(2-(4-bromo-5-methylthiophen-2-yl)-5-ethyl-6-...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1cc(Br)c(C)s1 Show InChI InChI=1S/C20H20BrN3O2S/c1-4-15-11(2)22-20(17-10-16(21)12(3)27-17)24-19(15)23-14-7-5-13(6-8-14)9-18(25)26/h5-8,10H,4,9H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557762
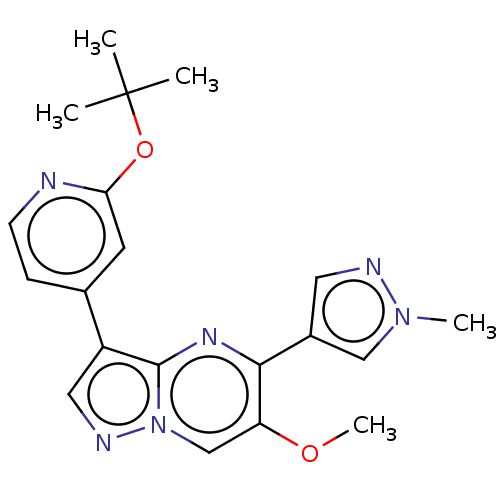
(CHEMBL4747422)Show SMILES COc1cn2ncc(-c3ccnc(OC(C)(C)C)c3)c2nc1-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557740

(CHEMBL4777071) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220714
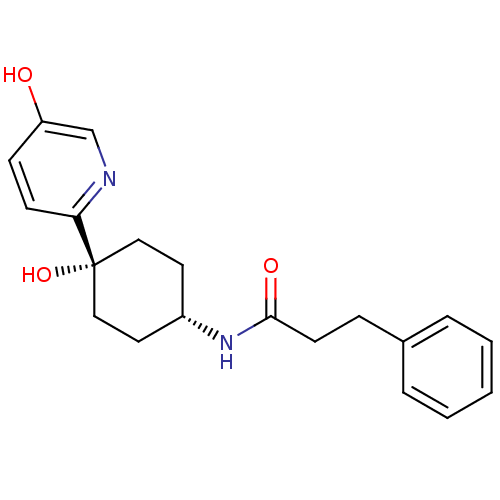
(CHEMBL237751 | N-((1s,4s)-4-hydroxy-4-(5-hydroxypy...)Show SMILES Oc1ccc(nc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(-10.78,-55,;-9.45,-54.23,;-9.45,-52.68,;-8.13,-51.91,;-6.79,-52.68,;-6.78,-54.21,;-8.1,-54.99,;-5.45,-51.91,;-5.46,-53.44,;-5.45,-50.37,;-4.13,-49.58,;-2.79,-50.37,;-2.79,-51.91,;-4.13,-52.68,;-1.45,-49.6,;-.12,-50.37,;-.12,-51.91,;1.23,-49.61,;2.55,-50.39,;3.89,-49.63,;5.21,-50.4,;6.55,-49.65,;6.56,-48.11,;5.24,-47.33,;3.9,-48.09,)| Show InChI InChI=1S/C20H24N2O3/c23-17-7-8-18(21-14-17)20(25)12-10-16(11-13-20)22-19(24)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,16,23,25H,6,9-13H2,(H,22,24)/t16-,20+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220726
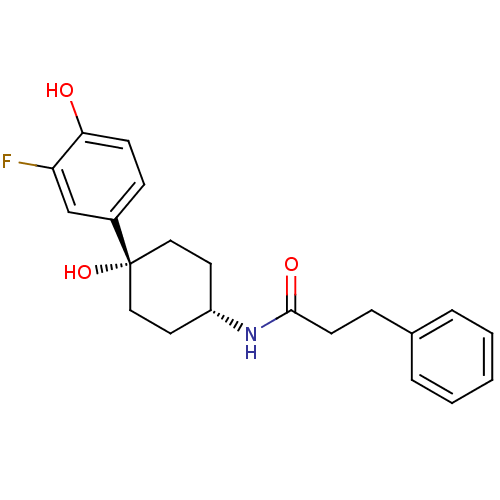
(CHEMBL237533 | N-((1s,4s)-4-(3-fluoro-4-hydroxyphe...)Show SMILES Oc1ccc(cc1F)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(12.39,-46.09,;13.72,-45.33,;15.07,-46.09,;16.39,-45.31,;16.38,-43.78,;15.04,-43.01,;13.72,-43.77,;12.38,-43,;17.72,-43,;17.71,-44.53,;17.72,-41.46,;19.04,-40.67,;20.38,-41.46,;20.38,-43,;19.04,-43.77,;21.72,-40.69,;23.05,-41.46,;23.05,-43.01,;24.4,-40.7,;25.72,-41.49,;27.06,-40.72,;28.37,-41.5,;29.72,-40.75,;29.73,-39.2,;28.41,-38.43,;27.07,-39.19,)| Show InChI InChI=1S/C21H24FNO3/c22-18-14-16(7-8-19(18)24)21(26)12-10-17(11-13-21)23-20(25)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,17,24,26H,6,9-13H2,(H,23,25)/t17-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346123

(2-(4-(5-ethyl-2-(5-fluorothiophen-2-yl)-6-methylpy...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1F)-c1ccc(F)s1 Show InChI InChI=1S/C19H17F2N3O2S/c1-3-12-10(2)22-19(15-6-7-16(21)27-15)24-18(12)23-14-5-4-11(8-13(14)20)9-17(25)26/h4-8H,3,9H2,1-2H3,(H,25,26)(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557741

(CHEMBL4750258) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346121
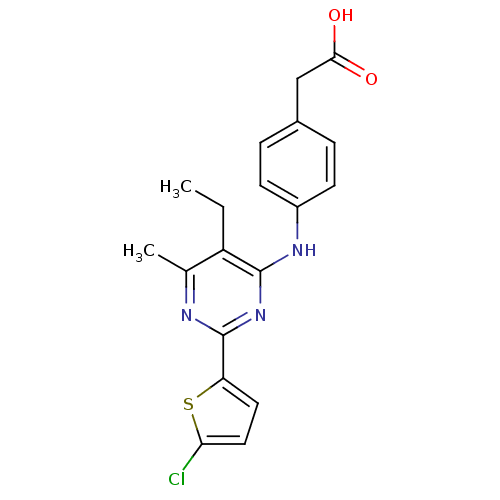
(2-(4-(2-(5-chlorothiophen-2-yl)-5-ethyl-6-methylpy...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(Cl)s1 Show InChI InChI=1S/C19H18ClN3O2S/c1-3-14-11(2)21-19(15-8-9-16(20)26-15)23-18(14)22-13-6-4-12(5-7-13)10-17(24)25/h4-9H,3,10H2,1-2H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50557768
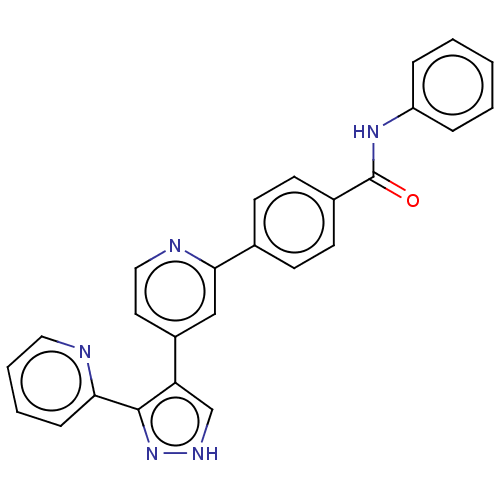
(CHEMBL4752410)Show SMILES O=C(Nc1ccccc1)c1ccc(cc1)-c1cc(ccn1)-c1c[nH]nc1-c1ccccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ALK5 (unknown origin) using biotin-labelled KKKVLTQMGSPSIRCSpSVS substrate in presence of [gamma33P] ATP measured after 40 min |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220729
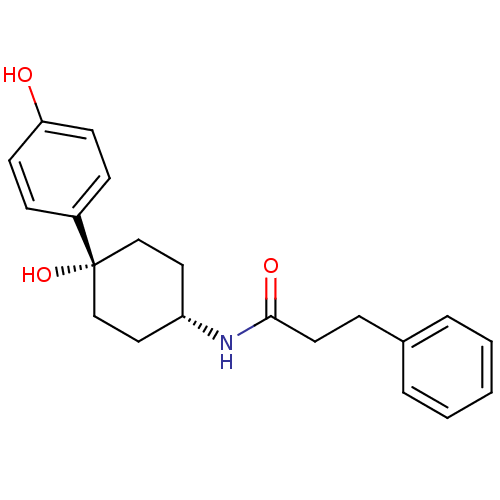
(CHEMBL237320 | N-((1s,4s)-4-hydroxy-4-(4-hydroxyph...)Show SMILES Oc1ccc(cc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(10.84,-24.52,;12.17,-23.76,;12.17,-22.2,;13.49,-21.43,;14.83,-22.2,;14.84,-23.74,;13.52,-24.52,;16.17,-21.43,;16.15,-22.96,;16.17,-19.89,;17.49,-19.1,;18.83,-19.89,;18.83,-21.43,;17.49,-22.2,;20.17,-19.12,;21.5,-19.89,;21.5,-21.44,;22.85,-19.13,;24.17,-19.91,;25.51,-19.15,;26.82,-19.93,;28.17,-19.18,;28.17,-17.63,;26.86,-16.86,;25.52,-17.61,)| Show InChI InChI=1S/C21H25NO3/c23-19-9-7-17(8-10-19)21(25)14-12-18(13-15-21)22-20(24)11-6-16-4-2-1-3-5-16/h1-5,7-10,18,23,25H,6,11-15H2,(H,22,24)/t18-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346120

(2-(4-(2-(5-bromothiophen-2-yl)-5-ethyl-6-methylpyr...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(Br)s1 Show InChI InChI=1S/C19H18BrN3O2S/c1-3-14-11(2)21-19(15-8-9-16(20)26-15)23-18(14)22-13-6-4-12(5-7-13)10-17(24)25/h4-9H,3,10H2,1-2H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220712
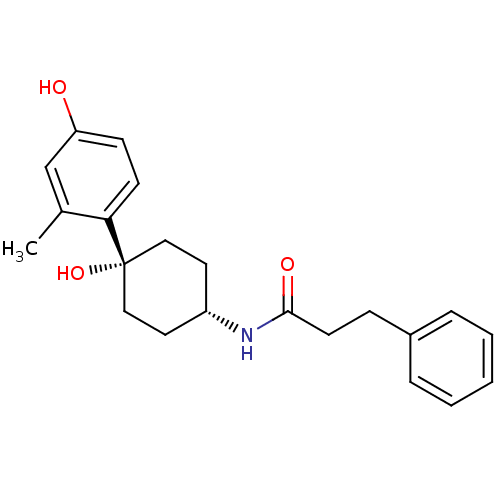
(CHEMBL395904 | N-((1s,4s)-4-hydroxy-4-(4-hydroxy-2...)Show SMILES Cc1cc(O)ccc1[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(34.08,-28.89,;34.09,-30.43,;32.77,-31.2,;32.76,-32.76,;31.44,-33.52,;34.11,-33.52,;35.44,-32.74,;35.42,-31.2,;36.76,-30.43,;36.75,-31.96,;36.76,-28.89,;38.08,-28.1,;39.43,-28.89,;39.43,-30.43,;38.08,-31.2,;40.76,-28.12,;42.1,-28.89,;42.1,-30.44,;43.44,-28.13,;44.76,-28.91,;46.1,-28.15,;47.42,-28.93,;48.77,-28.18,;48.77,-26.63,;47.45,-25.85,;46.12,-26.61,)| Show InChI InChI=1S/C22H27NO3/c1-16-15-19(24)8-9-20(16)22(26)13-11-18(12-14-22)23-21(25)10-7-17-5-3-2-4-6-17/h2-6,8-9,15,18,24,26H,7,10-14H2,1H3,(H,23,25)/t18-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220716
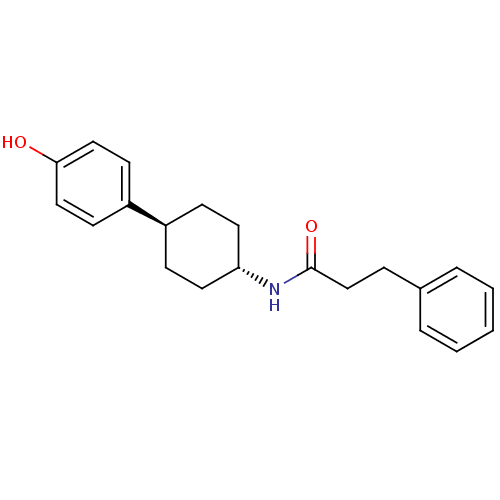
(CHEMBL236893 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:7.7,wD:10.14,(-10.14,-2.71,;-8.81,-1.94,;-8.81,-.39,;-7.49,.38,;-6.15,-.39,;-6.14,-1.92,;-7.46,-2.7,;-4.81,.38,;-4.81,1.92,;-3.49,2.71,;-2.14,1.92,;-2.14,.38,;-3.49,-.39,;-.81,2.69,;.52,1.92,;.52,.38,;1.87,2.68,;3.19,1.9,;4.54,2.66,;5.86,1.89,;7.19,2.65,;7.21,4.19,;5.87,4.97,;4.54,4.2,)| Show InChI InChI=1S/C21H25NO2/c23-20-13-9-18(10-14-20)17-7-11-19(12-8-17)22-21(24)15-6-16-4-2-1-3-5-16/h1-5,9-10,13-14,17,19,23H,6-8,11-12,15H2,(H,22,24)/t17-,19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346100

(4-(5-allyl-6-ethyl-2-phenylpyrimidin-4-ylamino)ben...)Show SMILES CCc1nc(nc(Nc2ccc(cc2)C(O)=O)c1CC=C)-c1ccccc1 Show InChI InChI=1S/C22H21N3O2/c1-3-8-18-19(4-2)24-20(15-9-6-5-7-10-15)25-21(18)23-17-13-11-16(12-14-17)22(26)27/h3,5-7,9-14H,1,4,8H2,2H3,(H,26,27)(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346119

(2-(4-(5-ethyl-6-methyl-2-(5-(methylthio)thiophen-2...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(SC)s1 Show InChI InChI=1S/C20H21N3O2S2/c1-4-15-12(2)21-20(16-9-10-18(26-3)27-16)23-19(15)22-14-7-5-13(6-8-14)11-17(24)25/h5-10H,4,11H2,1-3H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50557752

(CHEMBL4763122) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TGFbeta receptor 2 (unknown origin) using biotin-labelled TTLKDLIYDMTTSGSGSGLPLLVQRTIARTsubstrate in presence of [gamma33P] ATP measure... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00679
BindingDB Entry DOI: 10.7270/Q2PG1WD5 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220727
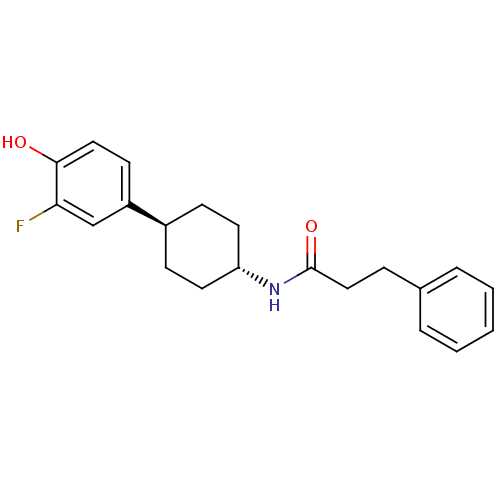
(CHEMBL237534 | N-((1r,4r)-4-(3-fluoro-4-hydroxyphe...)Show SMILES Oc1ccc(cc1F)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:8.8,wD:11.15,(31.75,-43.6,;33.08,-42.83,;34.43,-43.59,;35.75,-42.81,;35.74,-41.28,;34.4,-40.51,;33.08,-41.28,;31.74,-40.51,;37.08,-40.5,;37.08,-38.96,;38.4,-38.18,;39.74,-38.96,;39.74,-40.5,;38.4,-41.27,;41.08,-38.2,;42.41,-38.97,;42.41,-40.51,;43.76,-38.21,;45.08,-38.99,;46.42,-38.23,;47.74,-39,;49.08,-38.25,;49.09,-36.7,;47.77,-35.93,;46.43,-36.69,)| Show InChI InChI=1S/C21H24FNO2/c22-19-14-17(9-12-20(19)24)16-7-10-18(11-8-16)23-21(25)13-6-15-4-2-1-3-5-15/h1-5,9,12,14,16,18,24H,6-8,10-11,13H2,(H,23,25)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data