 Found 1135 hits with Last Name = 'simon' and Initial = 'a'
Found 1135 hits with Last Name = 'simon' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560224
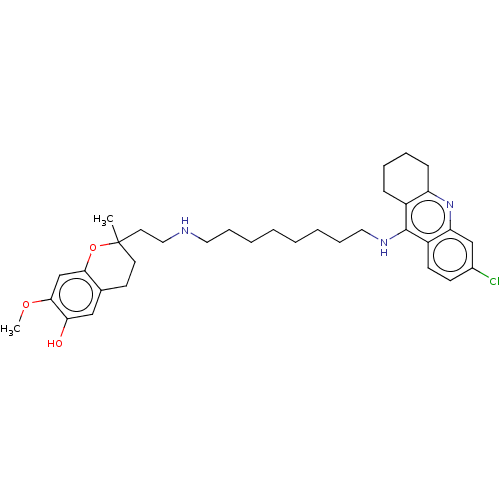
(CHEMBL4751100)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-inhibitor complex using varying lev... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560224

(CHEMBL4751100)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-substrate-inhibitor complex using v... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-inhibitor complex by Lineweaver-Burk d... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate-inhibitor complex by Linewea... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometr... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279

(CHEMBL3752467)Show SMILES C(CCCNCc1cc2ccccc2o1)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometric ... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50580216
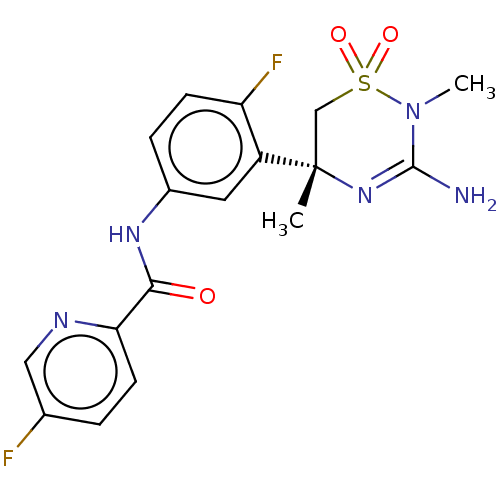
(MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...)Show SMILES CN1C(N)=N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r,c:3| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00931
BindingDB Entry DOI: 10.7270/Q2CF9V25 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50007801
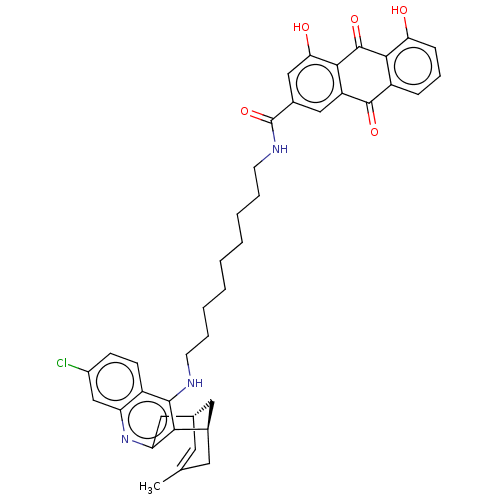
(CHEMBL3233832)Show SMILES Cl.[H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCNC(=O)c4cc(O)c5C(=O)c6c(O)cccc6C(=O)c5c4)c3[C@]([H])(CC(C)=C1)C2 |r,c:54| Show InChI InChI=1S/C41H42ClN3O5.ClH/c1-23-16-24-18-25(17-23)35-32(19-24)45-31-22-27(42)12-13-28(31)38(35)43-14-7-5-3-2-4-6-8-15-44-41(50)26-20-30-37(34(47)21-26)40(49)36-29(39(30)48)10-9-11-33(36)46;/h9-13,16,20-22,24-25,46-47H,2-8,14-15,17-19H2,1H3,(H,43,45)(H,44,50);1H/t24-,25+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-inhibitor complex by Li... |
J Med Chem 57: 2549-67 (2014)
Article DOI: 10.1021/jm401824w
BindingDB Entry DOI: 10.7270/Q2FX7BZ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50007801

(CHEMBL3233832)Show SMILES Cl.[H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCCCCCCNC(=O)c4cc(O)c5C(=O)c6c(O)cccc6C(=O)c5c4)c3[C@]([H])(CC(C)=C1)C2 |r,c:54| Show InChI InChI=1S/C41H42ClN3O5.ClH/c1-23-16-24-18-25(17-23)35-32(19-24)45-31-22-27(42)12-13-28(31)38(35)43-14-7-5-3-2-4-6-8-15-44-41(50)26-20-30-37(34(47)21-26)40(49)36-29(39(30)48)10-9-11-33(36)46;/h9-13,16,20-22,24-25,46-47H,2-8,14-15,17-19H2,1H3,(H,43,45)(H,44,50);1H/t24-,25+;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-substrate-inhibitor com... |
J Med Chem 57: 2549-67 (2014)
Article DOI: 10.1021/jm401824w
BindingDB Entry DOI: 10.7270/Q2FX7BZ9 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50019848
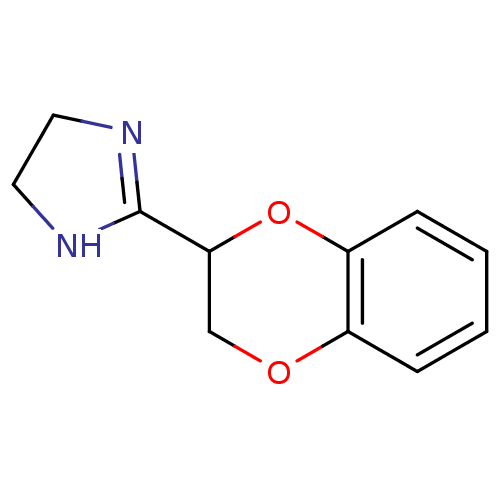
(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50027060
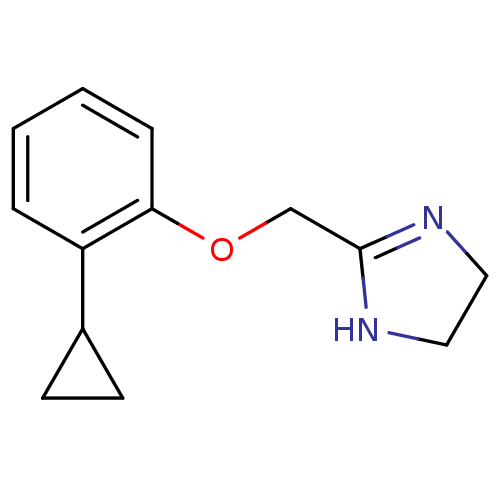
(2-(2-Cyclopropyl-phenoxymethyl)-4,5-dihydro-1H-imi...)Show InChI InChI=1S/C13H16N2O/c1-2-4-12(11(3-1)10-5-6-10)16-9-13-14-7-8-15-13/h1-4,10H,5-9H2,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50510823
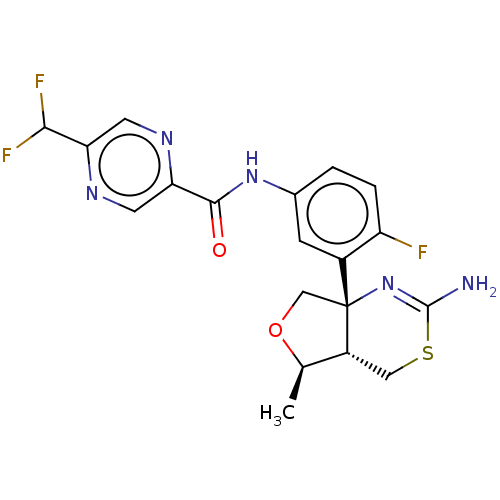
(E-2609 | E2609 | Elenbecestat)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H]2C)c1cc(NC(=O)c2cnc(cn2)C(F)F)ccc1F |r,c:5| Show InChI InChI=1S/C19H18F3N5O2S/c1-9-12-7-30-18(23)27-19(12,8-29-9)11-4-10(2-3-13(11)20)26-17(28)15-6-24-14(5-25-15)16(21)22/h2-6,9,12,16H,7-8H2,1H3,(H2,23,27)(H,26,28)/t9-,12-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00931
BindingDB Entry DOI: 10.7270/Q2CF9V25 |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM81804
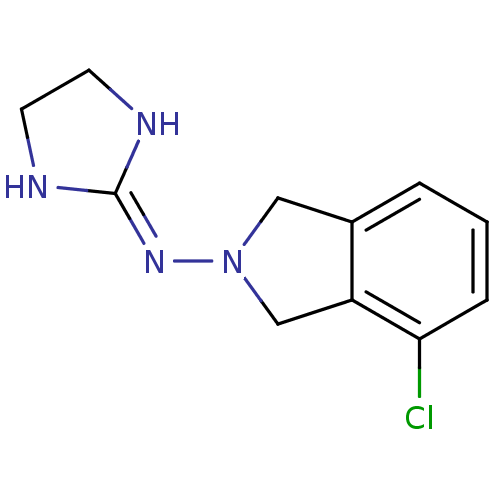
(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50001922
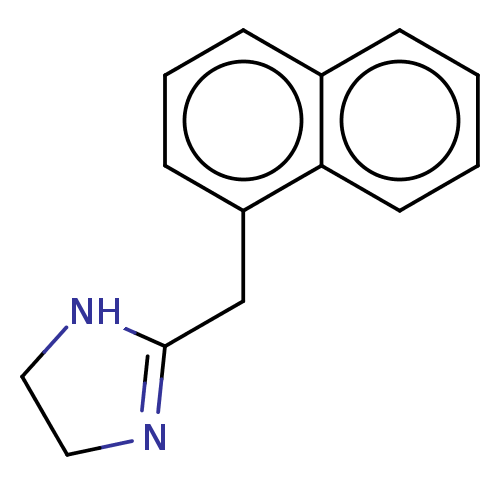
(2-Naphthalen-1-ylmethyl-4,5-dihydro-1H-imidazole; ...)Show InChI InChI=1S/C14H14N2/c1-2-7-13-11(4-1)5-3-6-12(13)10-14-15-8-9-16-14/h1-7H,8-10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50007674

((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...)Show SMILES C[C@@H]([C@H](O)c1ccc(O)cc1)N1CCC(Cc2ccccc2)CC1 Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM81982
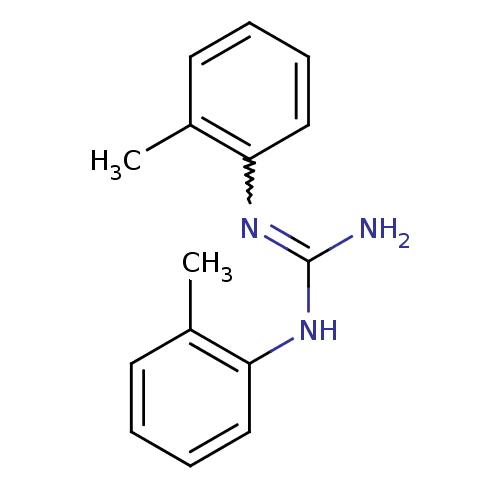
(CAS_97-39-2 | DITOLYLGUANIDINE | DTG | Di-o-tolylg...)Show InChI InChI=1S/C15H17N3/c1-11-7-3-5-9-13(11)17-15(16)18-14-10-6-4-8-12(14)2/h3-10H,1-2H3,(H3,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50062599
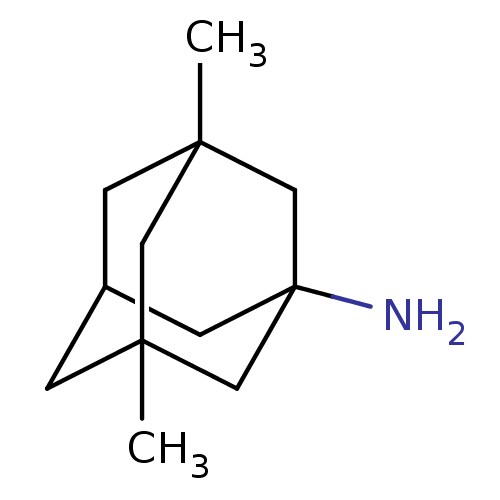
(3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...)Show SMILES CC12CC3CC(C)(C1)CC(N)(C3)C2 |TLB:7:1:4.5.8:11,10:9:4:2.7.1,0:1:4:8.9.11,THB:7:5:11:2.1.12,12:1:4:8.9.11,12:9:4:2.7.1,6:5:11:2.1.12| Show InChI InChI=1S/C12H21N/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10/h9H,3-8,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50502562
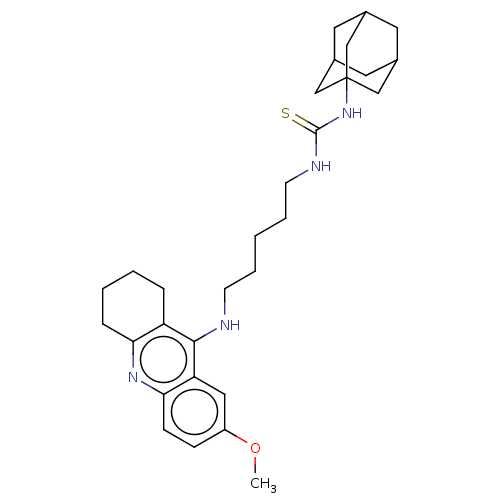
(CHEMBL4469066)Show SMILES COc1ccc2nc3CCCCc3c(NCCCCCNC(=S)NC34CC5CC(CC(C5)C3)C4)c2c1 |TLB:33:24:31:28.27.29,33:28:24.25.32:31,23:24:31:28.27.29,THB:29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C30H42N4OS/c1-35-23-9-10-27-25(16-23)28(24-7-3-4-8-26(24)33-27)31-11-5-2-6-12-32-29(36)34-30-17-20-13-21(18-30)15-22(14-20)19-30/h9-10,16,20-22H,2-8,11-15,17-19H2,1H3,(H,31,33)(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50016897
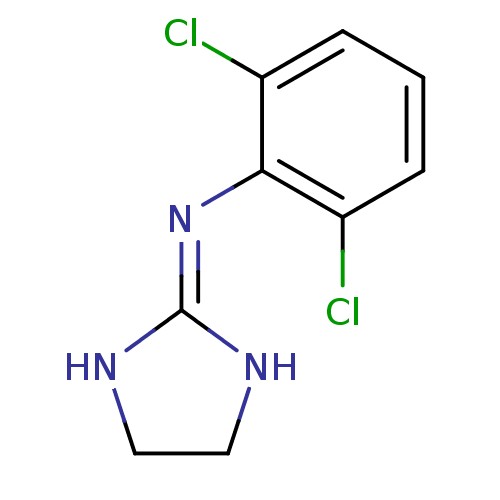
(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50000663
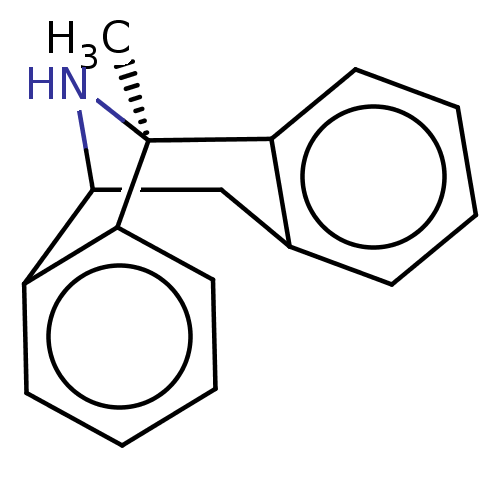
((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...)Show InChI InChI=1S/C16H15N/c1-16-13-8-4-2-6-11(13)10-15(17-16)12-7-3-5-9-14(12)16/h2-9,15,17H,10H2,1H3/t15?,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM22893
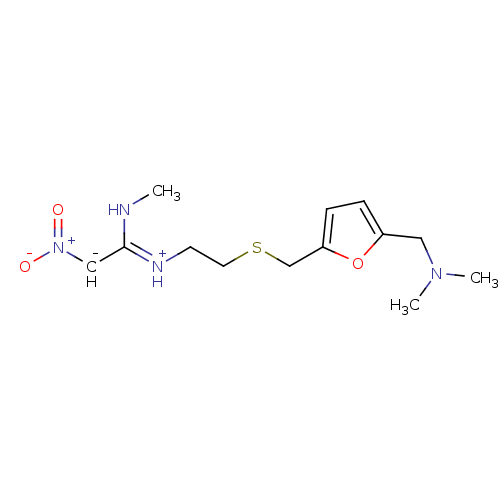
(CHEMBL512 | Ranitidine | ZANTAC | dimethyl[(5-{[(2...)Show SMILES CN\C([CH-][N+]([O-])=O)=[NH+]/CCSCc1ccc(CN(C)C)o1 Show InChI InChI=1S/C13H21N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9H,6-8,10H2,1-3H3,(H,14,15)/q-1/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50001028
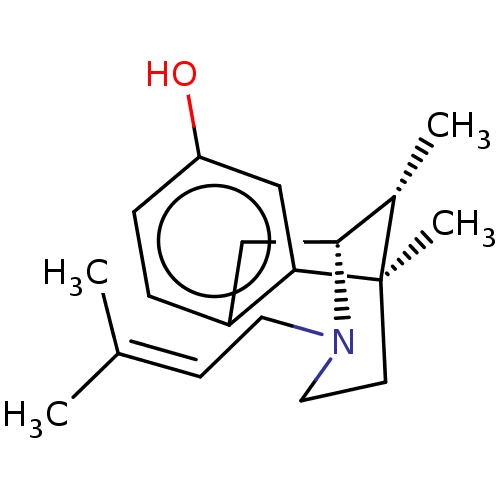
((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50001922

(2-Naphthalen-1-ylmethyl-4,5-dihydro-1H-imidazole; ...)Show InChI InChI=1S/C14H14N2/c1-2-7-13-11(4-1)5-3-6-12(13)10-14-15-8-9-16-14/h1-7H,8-10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50050093

((4-Chloro-6-methoxy-2-methyl-pyrimidin-5-yl)-imida...)Show SMILES [#6]-[#8]-c1nc(-[#6])nc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H12ClN5O/c1-5-13-7(10)6(8(14-5)16-2)15-9-11-3-4-12-9/h3-4H2,1-2H3,(H2,11,12,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50007674

((+)-erythro 4-[2-(4-Benzyl-piperidin-1-yl)-1-hydro...)Show SMILES C[C@@H]([C@H](O)c1ccc(O)cc1)N1CCC(Cc2ccccc2)CC1 Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50016897

(2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...)Show SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C9H9Cl2N3/c10-6-2-1-3-7(11)8(6)14-9-12-4-5-13-9/h1-3H,4-5H2,(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50027060

(2-(2-Cyclopropyl-phenoxymethyl)-4,5-dihydro-1H-imi...)Show InChI InChI=1S/C13H16N2O/c1-2-4-12(11(3-1)10-5-6-10)16-9-13-14-7-8-15-13/h1-4,10H,5-9H2,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM85213
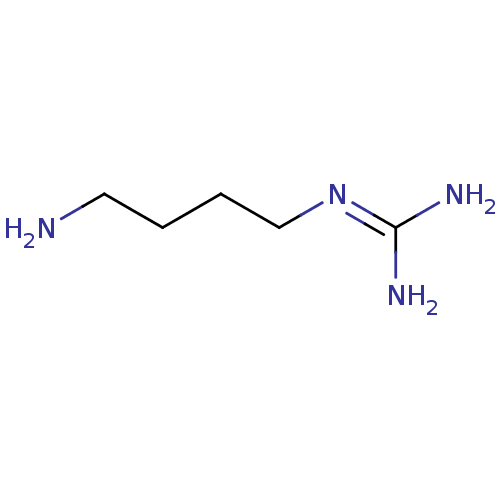
(Agmatine | CAS_306-60-5 | CHEMBL58343 | NSC_199 | ...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C5H14N4/c6-3-1-2-4-9-5(7)8/h1-4,6H2,(H4,7,8,9) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM50010617

((-)-3-(1-Propyl-piperidin-3-yl)-phenol | (S)-3-(1-...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Nischarin
(RAT) | BDBM85213

(Agmatine | CAS_306-60-5 | CHEMBL58343 | NSC_199 | ...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C5H14N4/c6-3-1-2-4-9-5(7)8/h1-4,6H2,(H4,7,8,9) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 170-7 (1998)
BindingDB Entry DOI: 10.7270/Q27H1H4B |
More data for this
Ligand-Target Pair | |
Sulfate anion transporter 1
(Mus musculus) | BDBM50400319
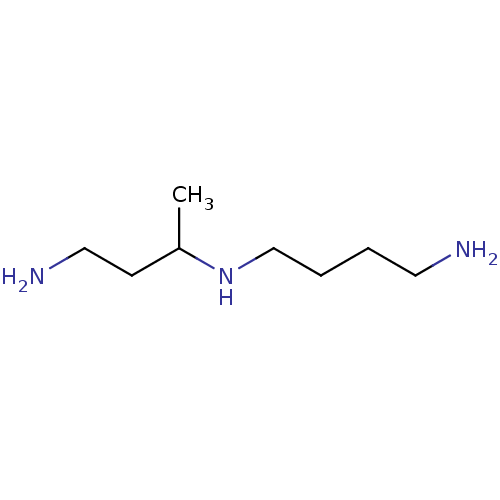
(CHEMBL2181335)Show InChI InChI=1S/C8H21N3/c1-8(4-6-10)11-7-3-2-5-9/h8,11H,2-7,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland
Curated by ChEMBL
| Assay Description
Competitive inhibition at mouse recombinant SSAT expressed in Escherichia coli strain BL21(DE3) |
J Med Chem 54: 4611-8 (2011)
Article DOI: 10.1021/jm200293r
BindingDB Entry DOI: 10.7270/Q2D79CJW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560224

(CHEMBL4751100)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560225

(CHEMBL4785201)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502563

(CHEMBL4471659)Show SMILES NC12CC3CC(F)(CC(C1)c1cc(NC(=O)CCCNc4c5CCCCc5nc5cc(Cl)ccc45)ccc31)C2 |TLB:10:8:3.2.4:38,11:10:9:5.7.38,THB:36:37:9:5.7.38,4:3:9:5.7.38,4:5:9:3.2.37.10| Show InChI InChI=1S/C32H36ClFN4O/c33-21-7-9-25-28(12-21)38-27-5-2-1-4-24(27)30(25)36-11-3-6-29(39)37-22-8-10-23-19-14-31(34)15-20(26(23)13-22)17-32(35,16-19)18-31/h7-10,12-13,19-20H,1-6,11,14-18,35H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579160
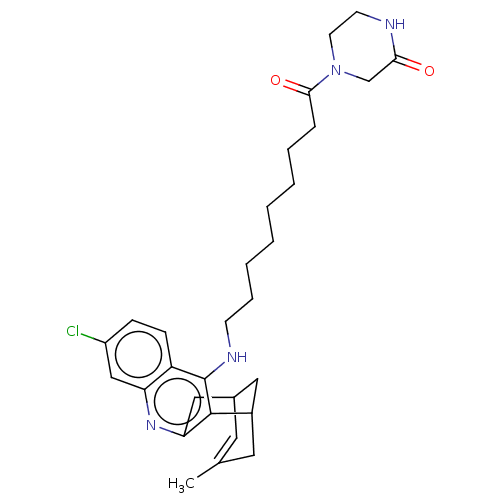
(CHEMBL4854913)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCC(=O)N1CCNC(=O)C1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560223
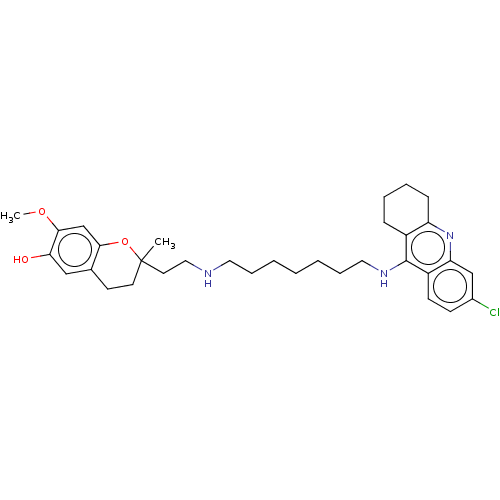
(CHEMBL4796461)Show SMILES COc1cc2OC(C)(CCNCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138463

(CHEMBL3753957)Show SMILES COc1cccc2cc(CN(CCCCCCNc3c4CCCCc4nc4ccccc34)Cc3cc4cccc(OC)c4o3)oc12 Show InChI InChI=1S/C39H43N3O4/c1-43-35-19-11-13-27-23-29(45-38(27)35)25-42(26-30-24-28-14-12-20-36(44-2)39(28)46-30)22-10-4-3-9-21-40-37-31-15-5-7-17-33(31)41-34-18-8-6-16-32(34)37/h5,7,11-15,17,19-20,23-24H,3-4,6,8-10,16,18,21-22,25-26H2,1-2H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50138438

(CHEMBL3752969)Show SMILES COc1cccc2cc(CNCCCCCCNc3c4CCCCc4nc4ccccc34)oc12 Show InChI InChI=1S/C29H35N3O2/c1-33-27-16-10-11-21-19-22(34-29(21)27)20-30-17-8-2-3-9-18-31-28-23-12-4-6-14-25(23)32-26-15-7-5-13-24(26)28/h4,6,10-12,14,16,19,30H,2-3,5,7-9,13,15,17-18,20H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
J Med Chem 59: 114-31 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01119
BindingDB Entry DOI: 10.7270/Q2S75J5X |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50236331
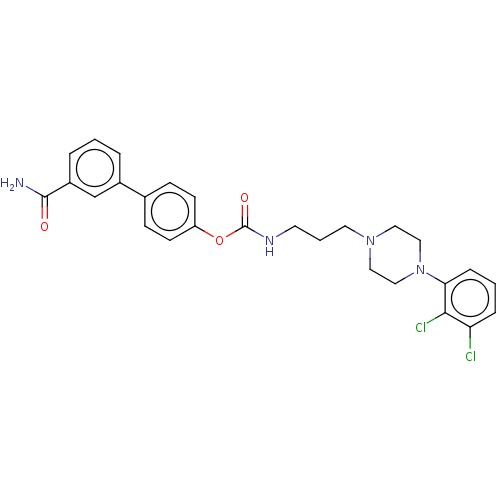
(CHEMBL4091498)Show SMILES NC(=O)c1cccc(c1)-c1ccc(OC(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C27H28Cl2N4O3/c28-23-6-2-7-24(25(23)29)33-16-14-32(15-17-33)13-3-12-31-27(35)36-22-10-8-19(9-11-22)20-4-1-5-21(18-20)26(30)34/h1-2,4-11,18H,3,12-17H2,(H2,30,34)(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... |
J Med Chem 60: 2287-2304 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01578
BindingDB Entry DOI: 10.7270/Q29S1T9P |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50236333
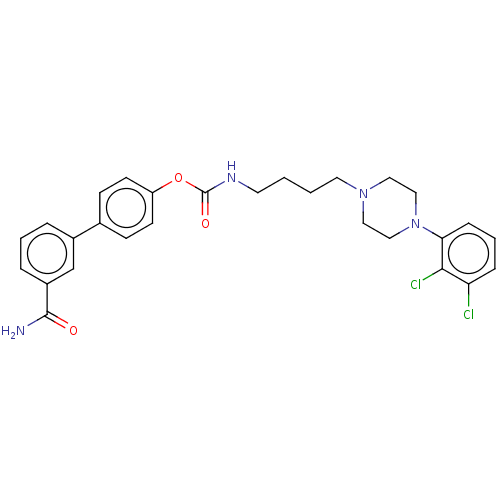
(CHEMBL4092052)Show SMILES NC(=O)c1cccc(c1)-c1ccc(OC(=O)NCCCCN2CCN(CC2)c2cccc(Cl)c2Cl)cc1 Show InChI InChI=1S/C28H30Cl2N4O3/c29-24-7-4-8-25(26(24)30)34-17-15-33(16-18-34)14-2-1-13-32-28(36)37-23-11-9-20(10-12-23)21-5-3-6-22(19-21)27(31)35/h3-12,19H,1-2,13-18H2,(H2,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... |
J Med Chem 60: 2287-2304 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01578
BindingDB Entry DOI: 10.7270/Q29S1T9P |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50236330
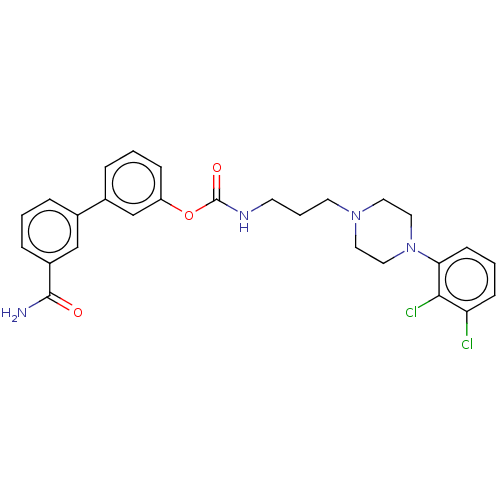
(CHEMBL4070196)Show SMILES NC(=O)c1cccc(c1)-c1cccc(OC(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)c1 Show InChI InChI=1S/C27H28Cl2N4O3/c28-23-9-3-10-24(25(23)29)33-15-13-32(14-16-33)12-4-11-31-27(35)36-22-8-2-6-20(18-22)19-5-1-7-21(17-19)26(30)34/h1-3,5-10,17-18H,4,11-16H2,(H2,30,34)(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... |
J Med Chem 60: 2287-2304 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01578
BindingDB Entry DOI: 10.7270/Q29S1T9P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM41542
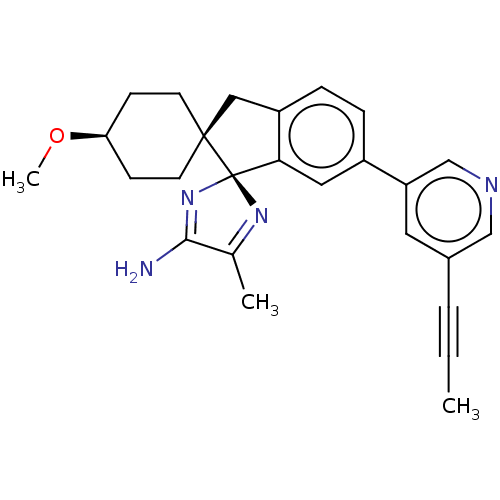
(US8865911, 122)Show SMILES CO[C@H]1CC[C@@]2(Cc3ccc(cc3[C@@]22N=C(C)C(N)=N2)-c2cncc(c2)C#CC)CC1 |r,wU:13.15,wD:5.5,2.1,c:20,t:16,(7.78,-3.08,;7.01,-1.75,;5.47,-1.75,;4.7,-.41,;3.16,-.41,;2.39,-1.75,;1.48,-2.99,;.02,-2.52,;-1.32,-3.29,;-2.65,-2.52,;-2.65,-.98,;-1.32,-.21,;.02,-.98,;1.48,-.5,;.24,.4,;.71,1.87,;-.06,3.2,;2.25,1.87,;3.02,3.2,;2.73,.4,;-3.98,-.21,;-5.32,-.98,;-6.65,-.21,;-6.65,1.33,;-5.32,2.1,;-3.98,1.33,;-5.32,3.64,;-5.32,5.18,;-5.32,6.72,;3.16,-3.08,;4.7,-3.08,)| Show InChI InChI=1S/C17H17FN4O2/c1-2-3-15-8-16(21-24-15)17(23)20-14-9-19-22(11-14)10-12-4-6-13(18)7-5-12/h4-9,11H,2-3,10H2,1H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00931
BindingDB Entry DOI: 10.7270/Q2CF9V25 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50236343

(CHEMBL4086944)Show SMILES NC(=O)c1cccc(c1)-c1ccc(OC(=O)NC\C=C\CN2CCN(CC2)c2cccc(Cl)c2Cl)cc1F Show InChI InChI=1S/C28H27Cl2FN4O3/c29-23-7-4-8-25(26(23)30)35-15-13-34(14-16-35)12-2-1-11-33-28(37)38-21-9-10-22(24(31)18-21)19-5-3-6-20(17-19)27(32)36/h1-10,17-18H,11-16H2,(H2,32,36)(H,33,37)/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... |
J Med Chem 60: 2287-2304 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01578
BindingDB Entry DOI: 10.7270/Q29S1T9P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data