

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267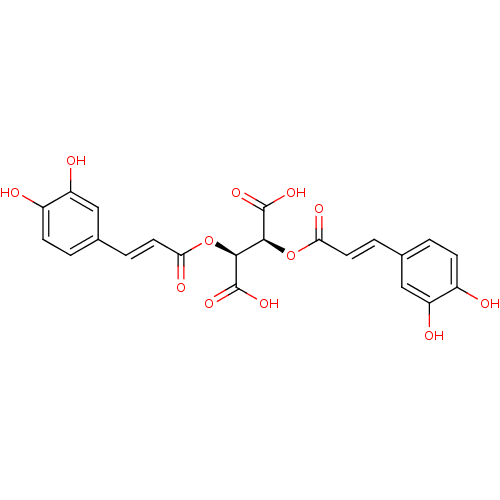 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865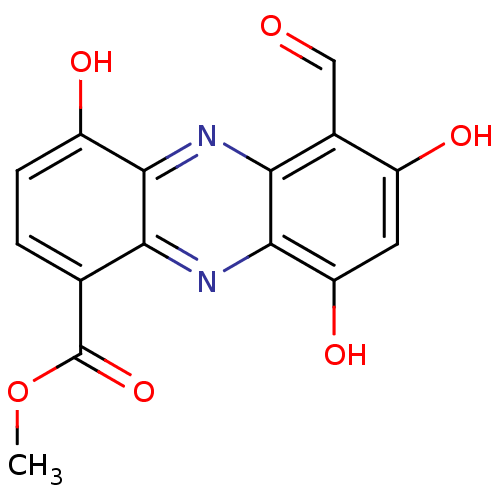 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485657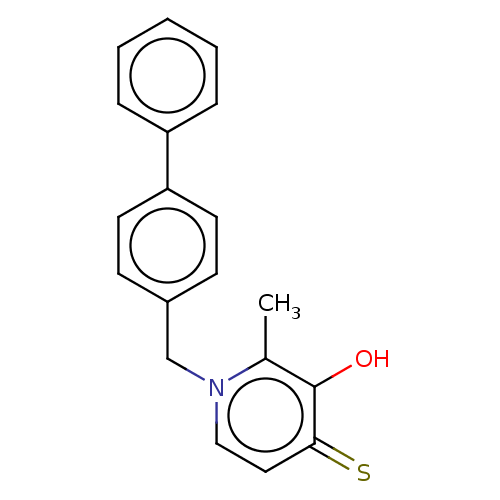 (CHEMBL2146996) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485648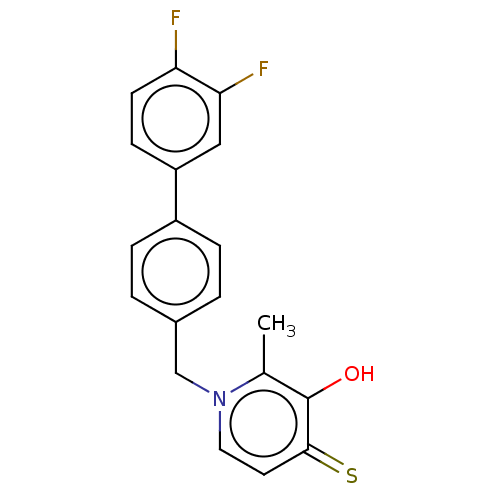 (CHEMBL2146995) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485651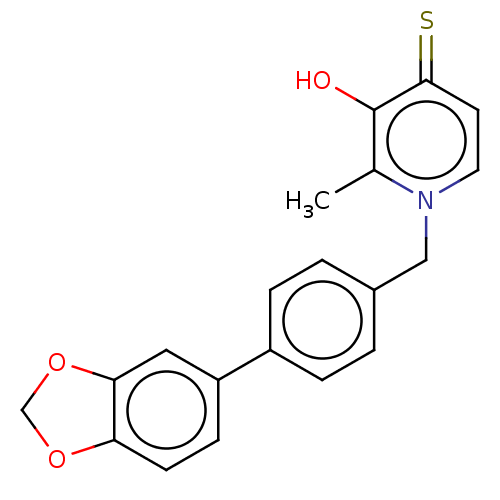 (CHEMBL2146997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485646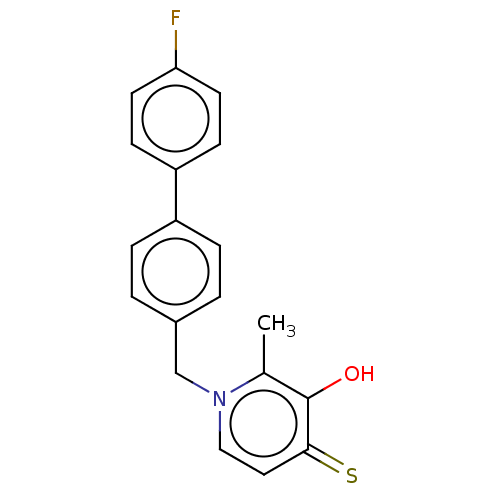 (CHEMBL2146998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485653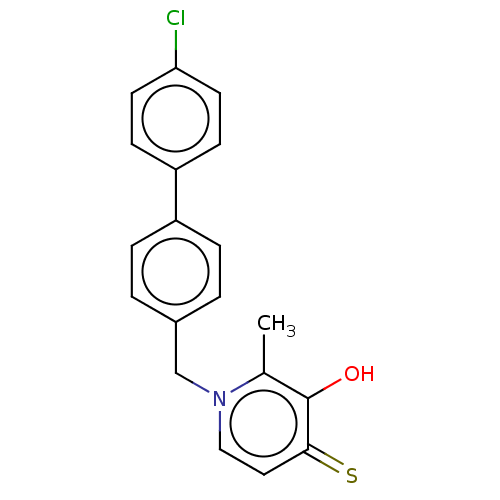 (CHEMBL2147001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485647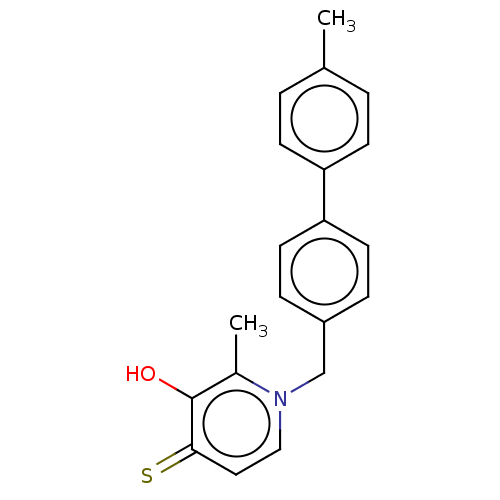 (CHEMBL2146999) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485654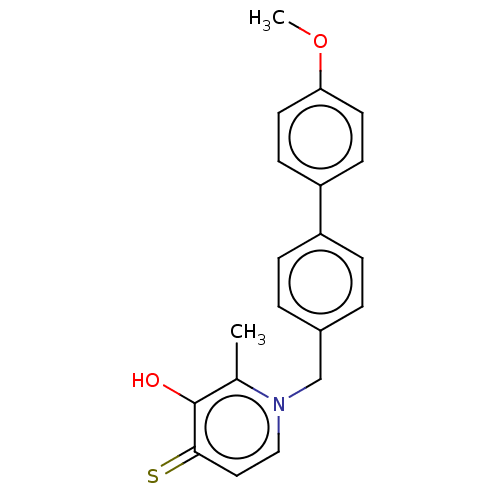 (CHEMBL2147000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50015094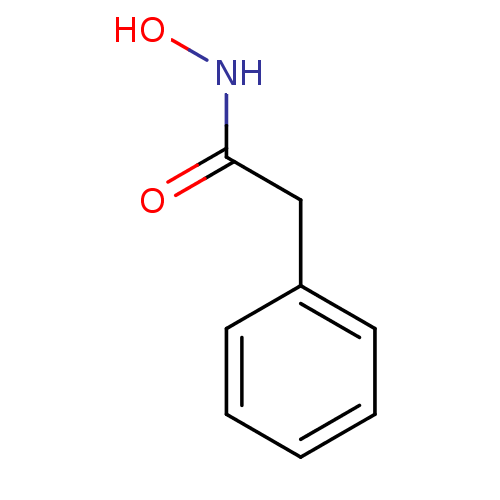 (CHEMBL152665 | N-Hydroxy-2-phenyl-acetamide | N-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485655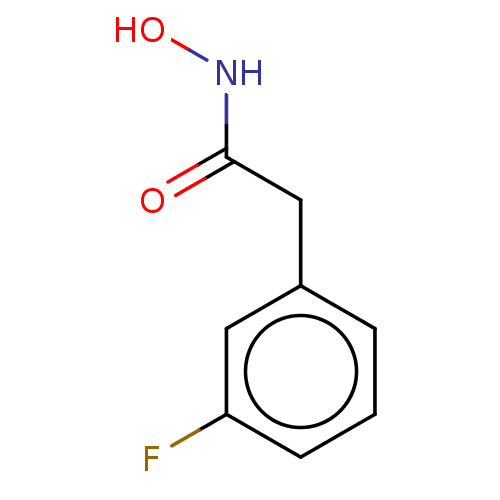 (CHEMBL2146994) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485649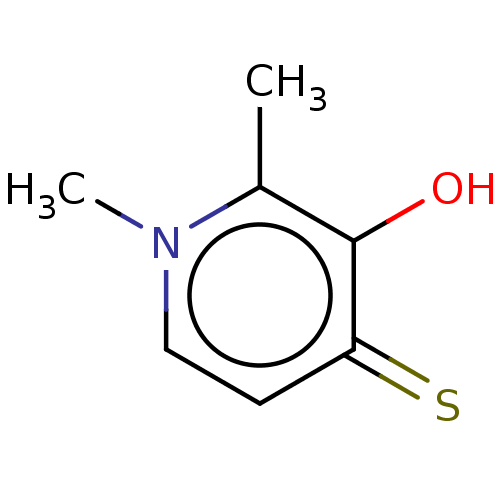 (CHEMBL1650620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485656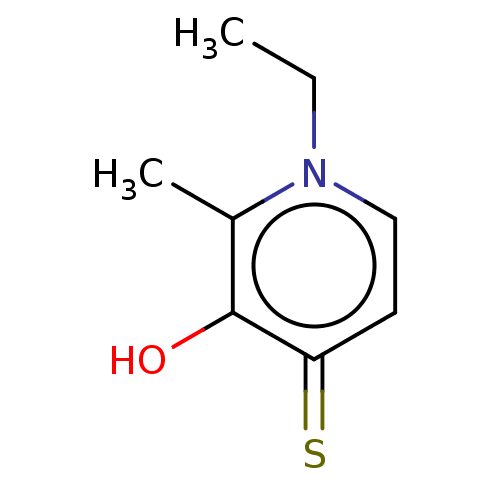 (CHEMBL1650624) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485652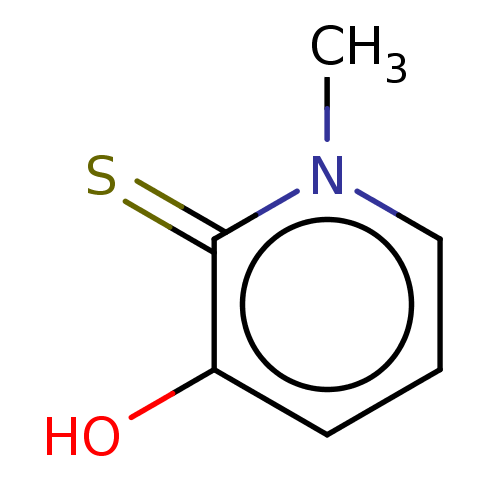 (CHEMBL1650621) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485650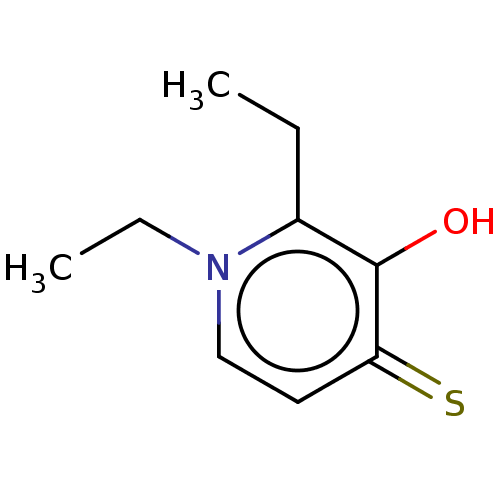 (CHEMBL1650623) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||