

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042607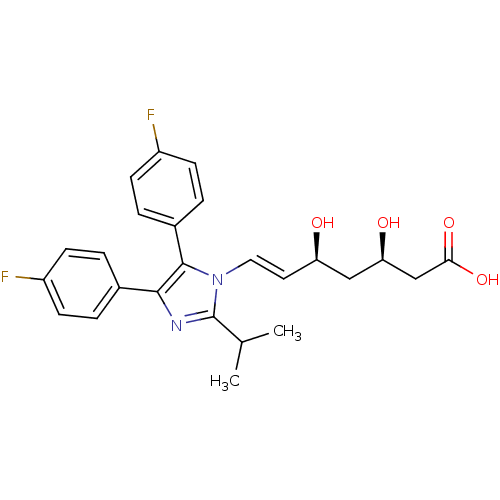 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042631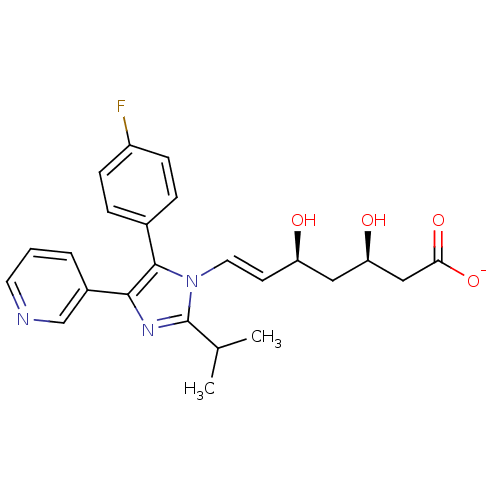 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042625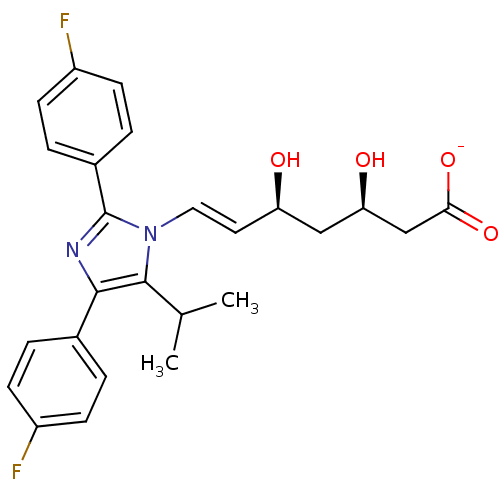 ((E)-(3R,5S)-7-[2,4-Bis-(4-fluoro-phenyl)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281061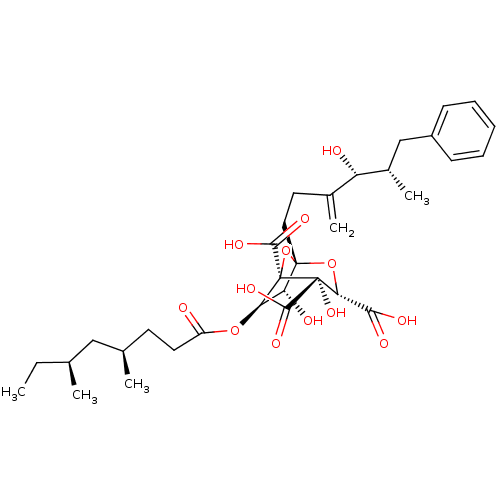 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042615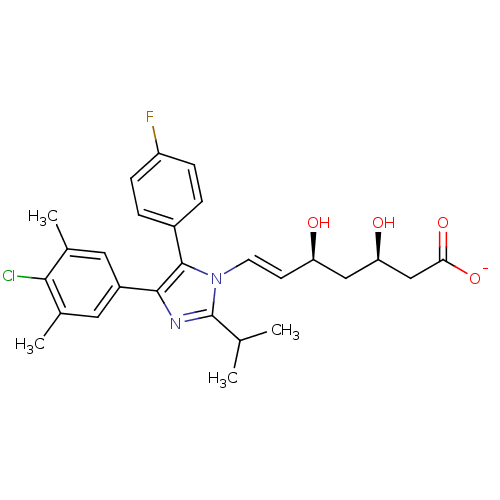 ((E)-(3R,5S)-7-[4-(4-Chloro-3,5-dimethyl-phenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042614 (CHEMBL120932 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042620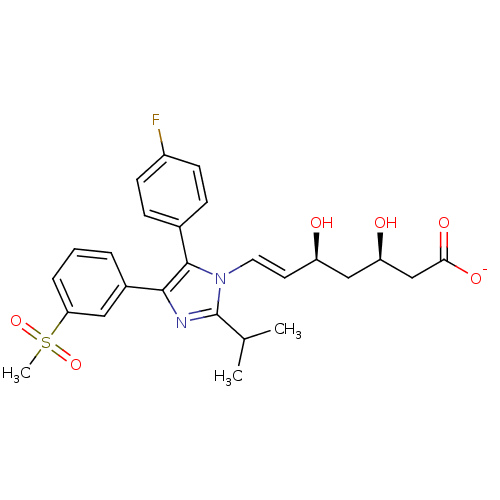 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042629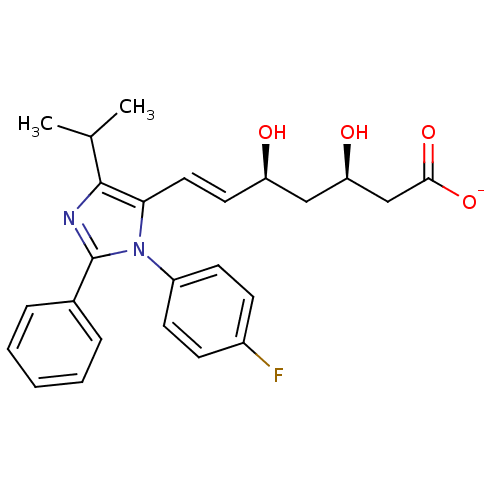 (CHEMBL121309 | Sodium; 7-[3-(4-fluoro-phenyl)-5-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051875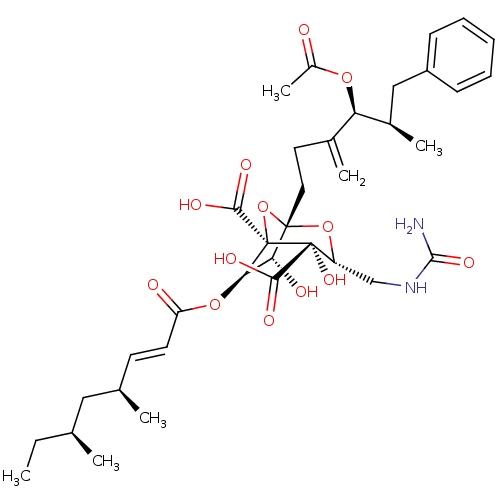 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287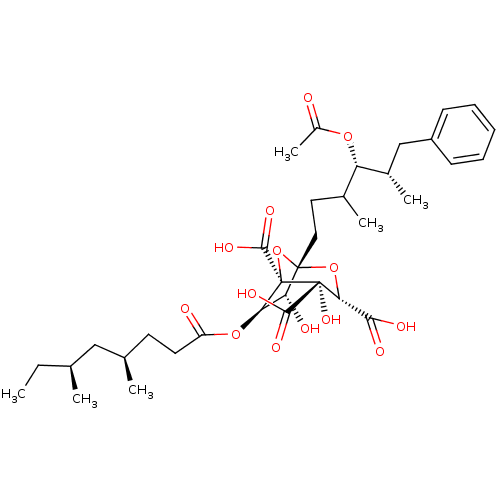 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042601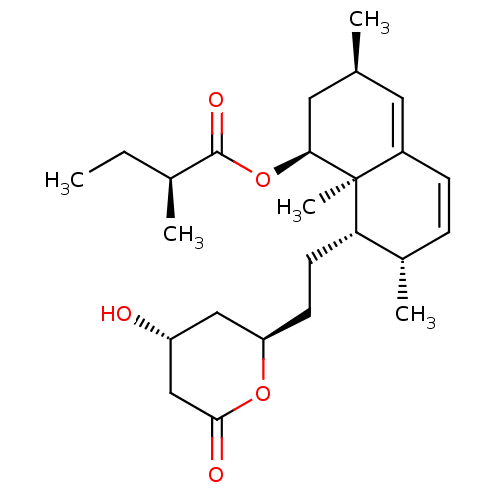 (2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042622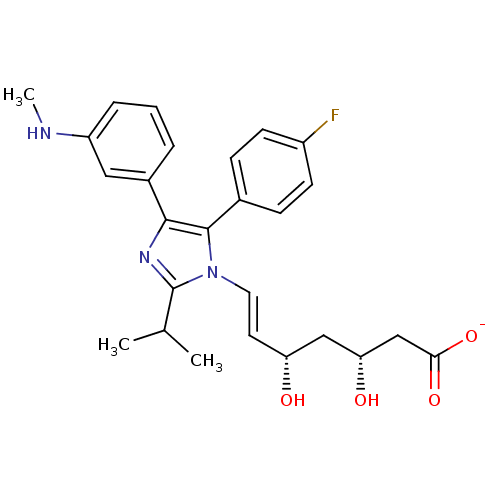 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051879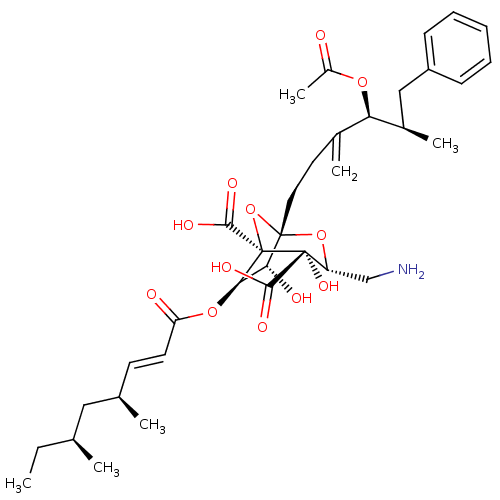 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281068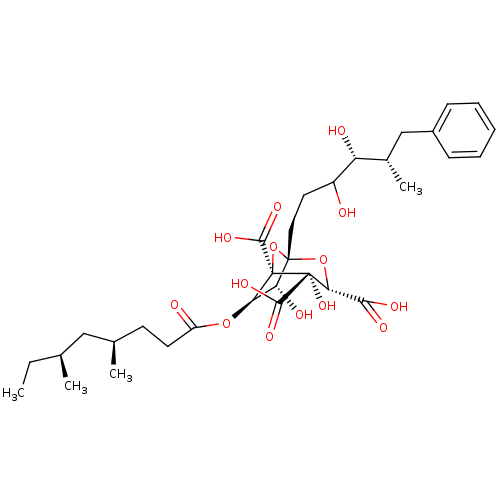 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-3,4-Dihydroxy-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042612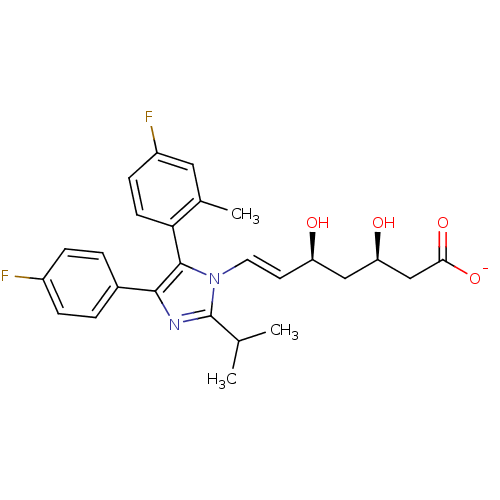 (CHEMBL121610 | Sodium; 7-[5-(4-fluoro-2-methyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033185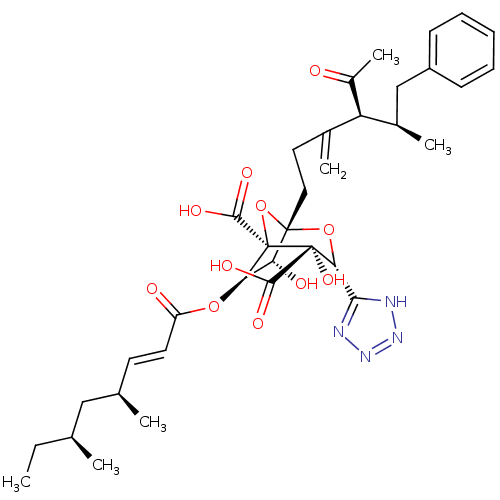 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281085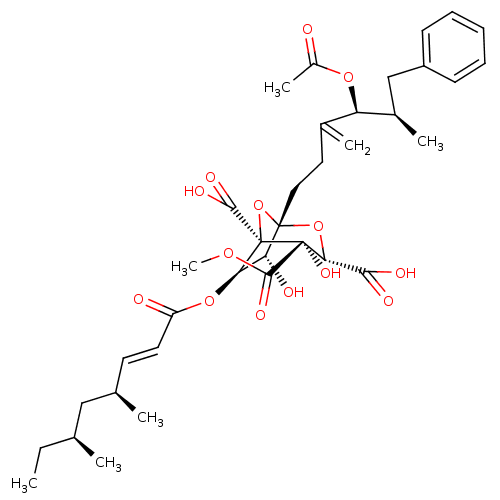 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity measured against rat squalene synthase(SQS) enzyme | Bioorg Med Chem Lett 3: 2541-2546 (1993) Article DOI: 10.1016/S0960-894X(01)80713-7 BindingDB Entry DOI: 10.7270/Q26Q1XQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285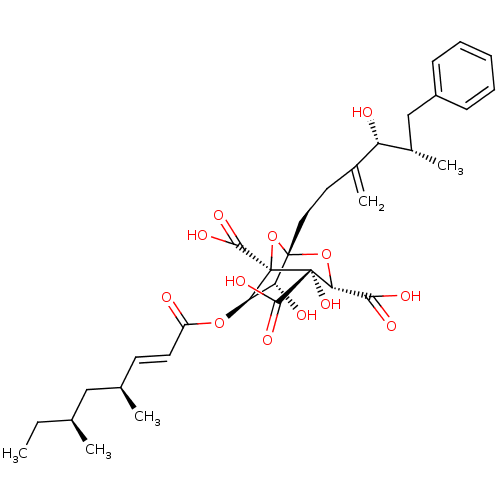 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Squalene synthase activity in juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281062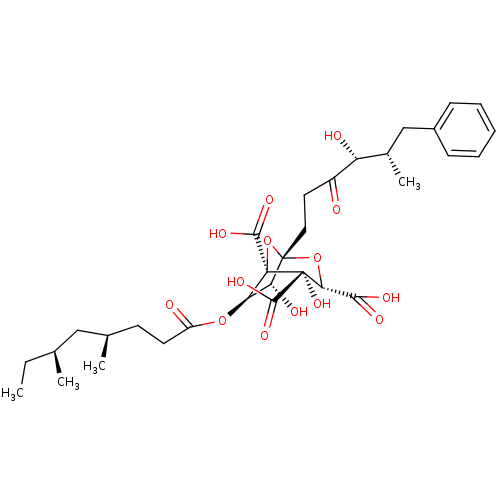 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of Squalene synthase activity, measured using juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042611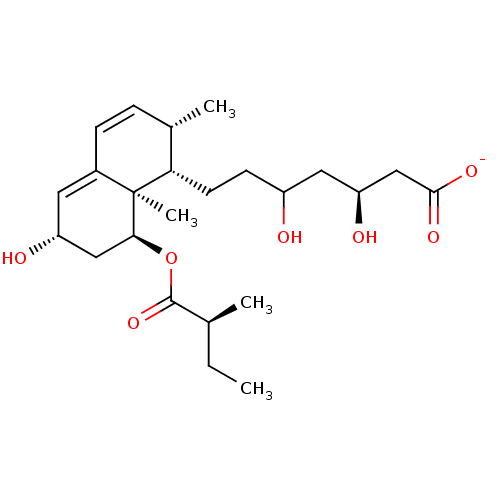 (CHEMBL333003 | Sodium; 3,5-dihydroxy-7-[6-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037285 ((1S,3S,4S,5R,6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037286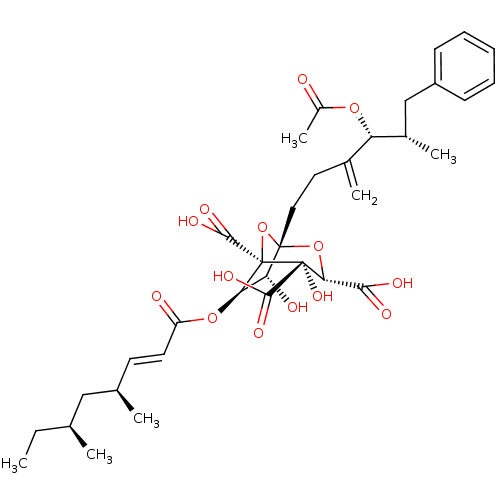 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50051873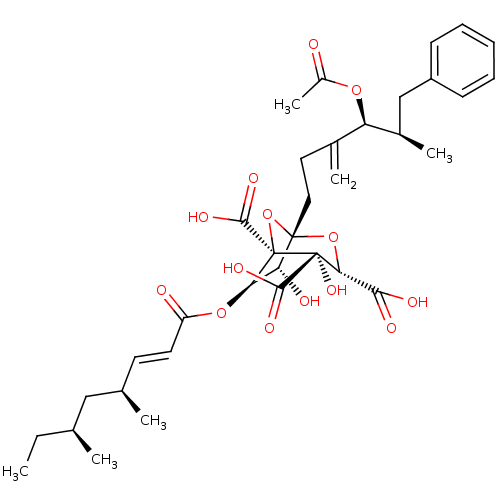 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of compound was measured against Candida squalene synthase(SQS) enzyme | Bioorg Med Chem Lett 3: 2541-2546 (1993) Article DOI: 10.1016/S0960-894X(01)80713-7 BindingDB Entry DOI: 10.7270/Q26Q1XQP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051872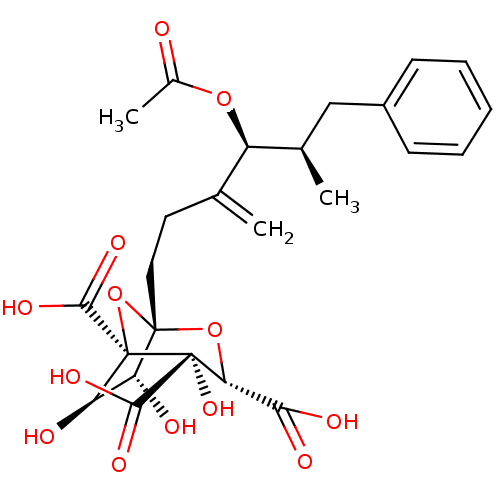 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity measured against rat squalene synthase(SQS) enzyme | Bioorg Med Chem Lett 3: 2541-2546 (1993) Article DOI: 10.1016/S0960-894X(01)80713-7 BindingDB Entry DOI: 10.7270/Q26Q1XQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281078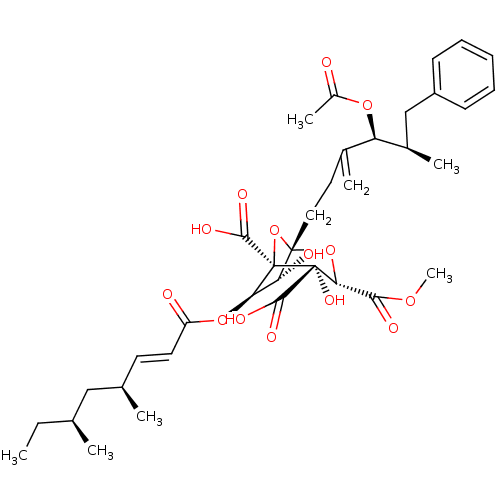 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat squalene synthase | Bioorg Med Chem Lett 4: 1931-1936 (1994) Article DOI: 10.1016/S0960-894X(01)80537-0 BindingDB Entry DOI: 10.7270/Q2D50MW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281078 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity measured against rat squalene synthase(SQS) enzyme | Bioorg Med Chem Lett 3: 2541-2546 (1993) Article DOI: 10.1016/S0960-894X(01)80713-7 BindingDB Entry DOI: 10.7270/Q26Q1XQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042616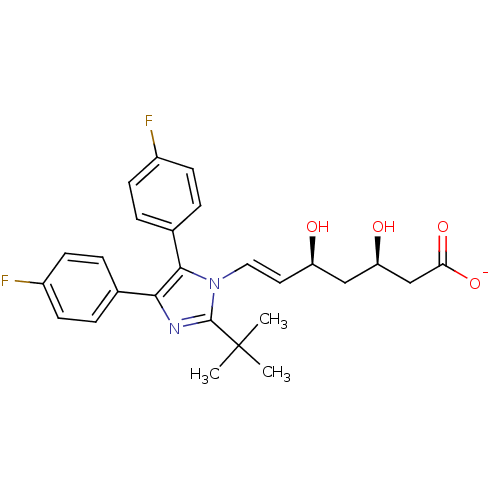 ((E)-(3R,5S)-7-[2-tert-Butyl-4,5-bis-(4-fluoro-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033196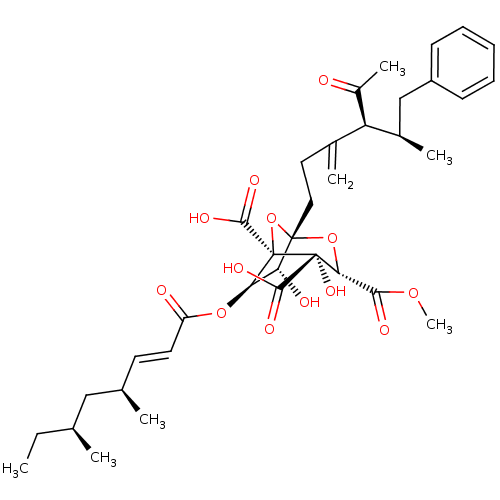 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037287 ((1S,3S,4S,5R,6R,7R)-1-((4S,5S)-4-Acetoxy-3,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042643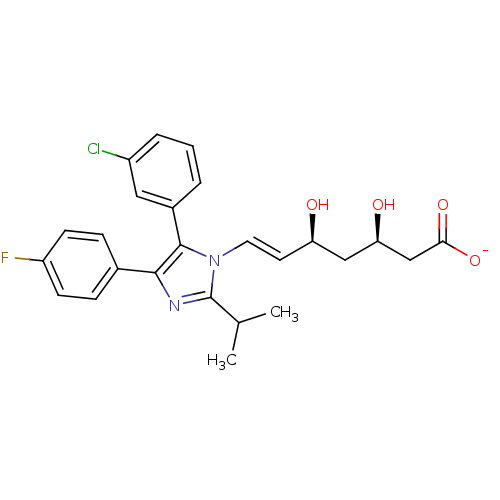 ((E)-(3R,5S)-7-[5-(3-Chloro-phenyl)-4-(4-fluoro-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042634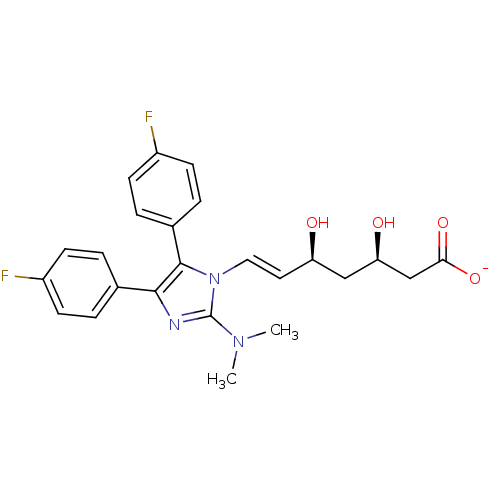 ((E)-(3R,5S)-7-[2-Dimethylamino-4,5-bis-(4-fluoro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037279 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033184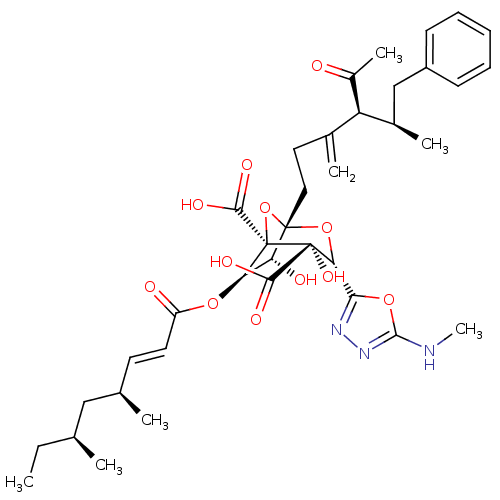 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051874 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051866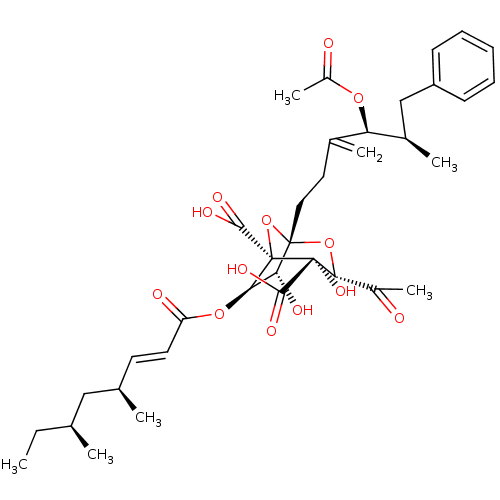 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051873 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037286 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Squalene synthase activity in juvenile male rat liver microsomes | Bioorg Med Chem Lett 3: 2527-2532 (1993) Article DOI: 10.1016/S0960-894X(01)80710-1 BindingDB Entry DOI: 10.7270/Q2HM58CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037286 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051873 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity measured against rat squalene synthase(SQS) enzyme | Bioorg Med Chem Lett 3: 2541-2546 (1993) Article DOI: 10.1016/S0960-894X(01)80713-7 BindingDB Entry DOI: 10.7270/Q26Q1XQP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033199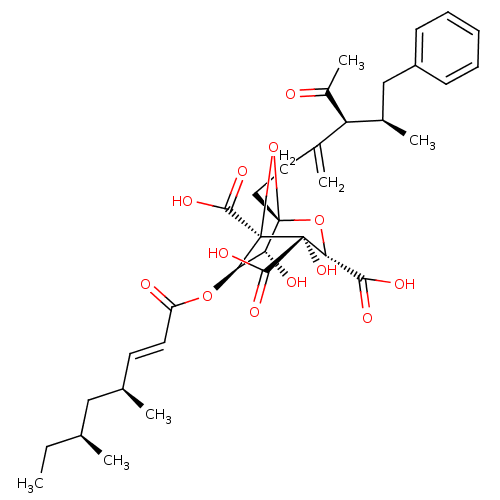 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051873 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat squalene synthase | Bioorg Med Chem Lett 4: 1931-1936 (1994) Article DOI: 10.1016/S0960-894X(01)80537-0 BindingDB Entry DOI: 10.7270/Q2D50MW5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037291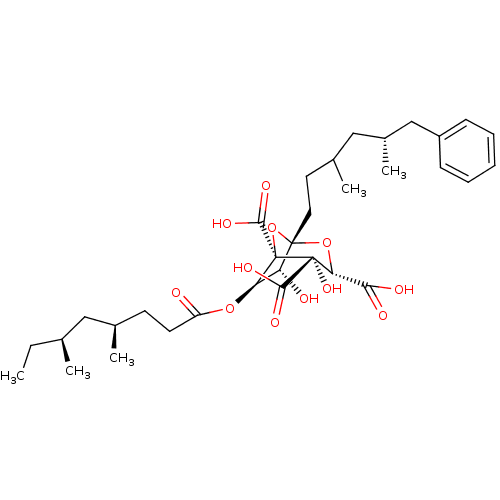 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051867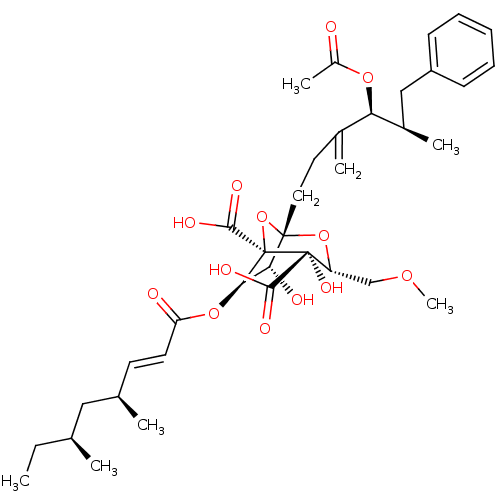 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042628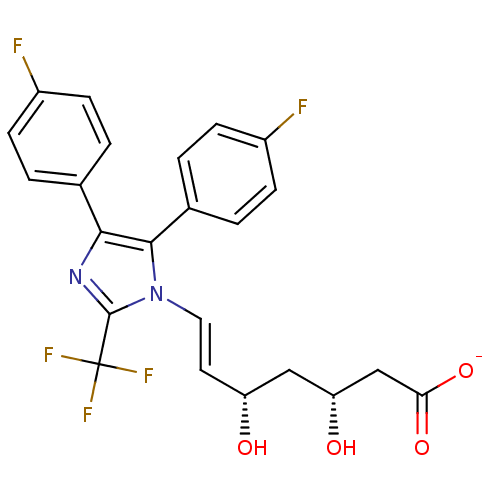 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50037289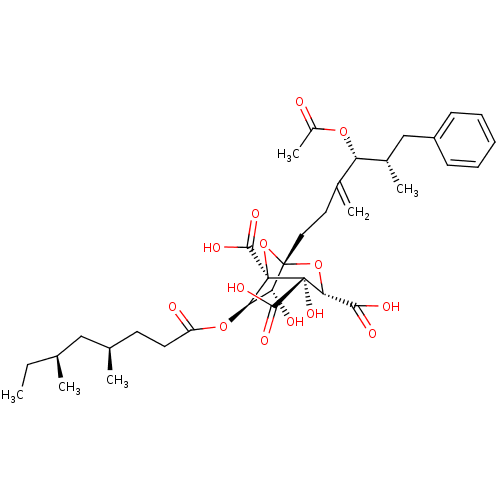 ((1S,3S,4S,5R,6R,7R)-1-((4R,5S)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Inhibition of juvenile male rat liver microsomal squalene synthase | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50037291 ((1S,3S,4S,5R,6R,7R)-6-((4R,6S)-4,6-Dimethyl-octano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Candida albicans 2005E microsomal SQS | J Med Chem 37: 3274-81 (1994) BindingDB Entry DOI: 10.7270/Q2X06633 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051864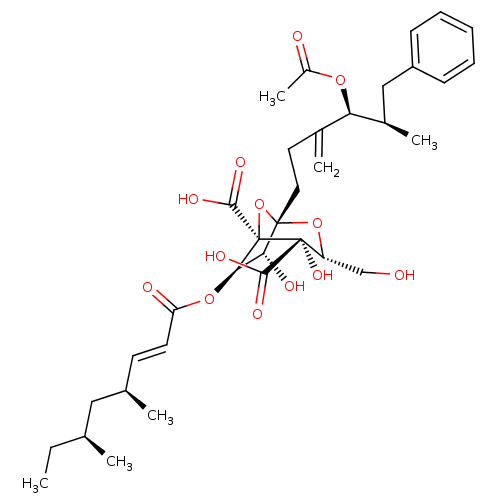 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 162 total ) | Next | Last >> |