

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50174298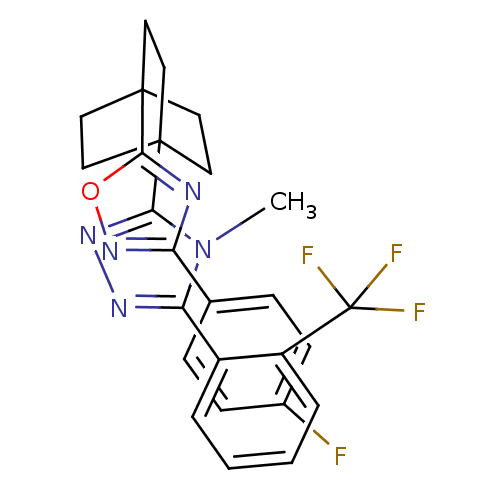 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340378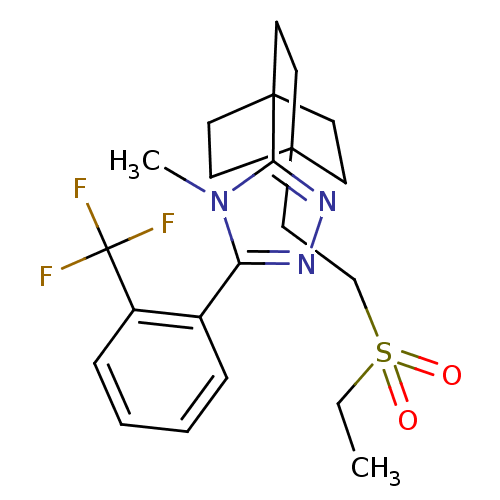 (3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50435691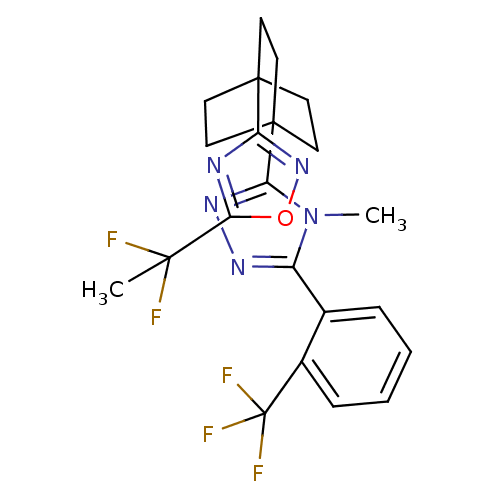 (CHEMBL2391968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340386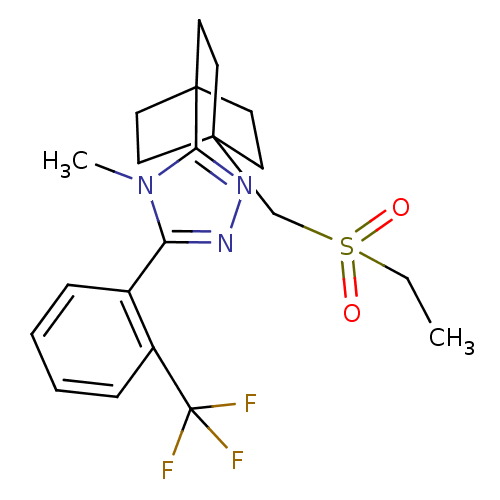 (3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273535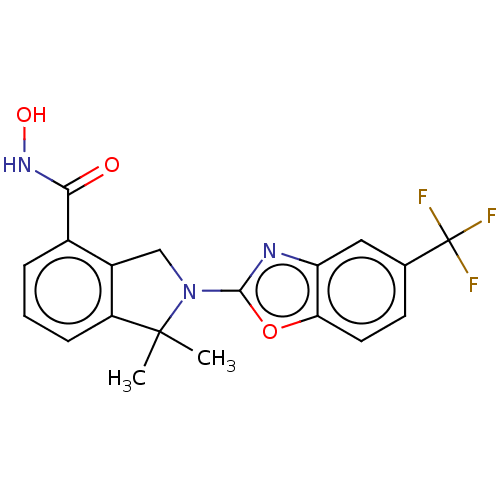 (CHEMBL4130288 | US11535607, Example 18-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273525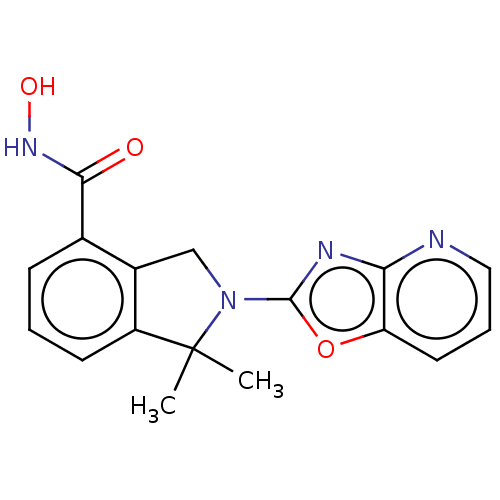 (CHEMBL4128972 | US11535607, Example 18-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273522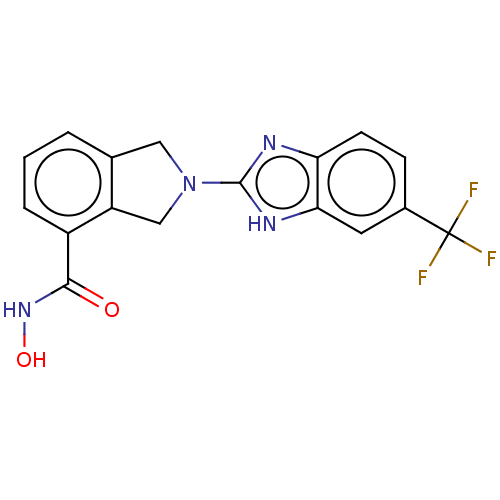 (CHEMBL4127743 | US11535607, Example 12-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273537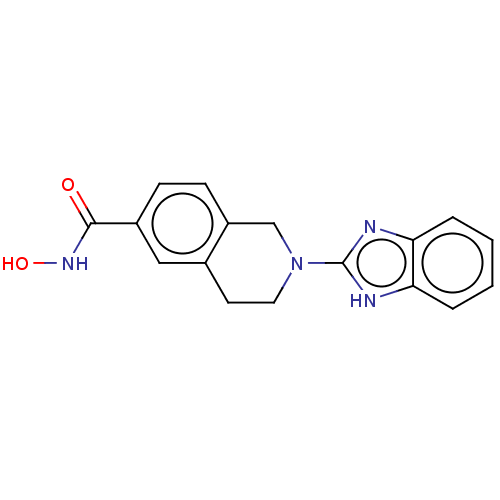 (CHEMBL4128164 | US11535607, Example 50-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273553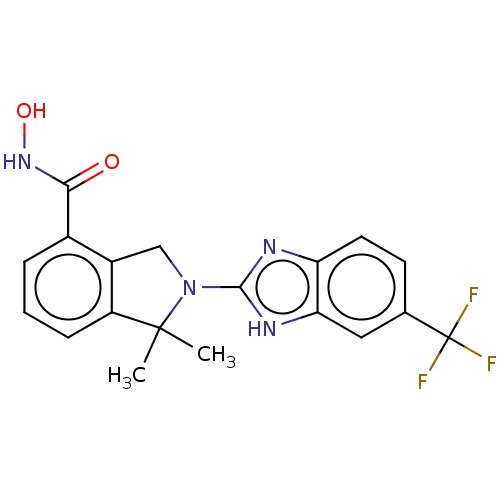 (CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273520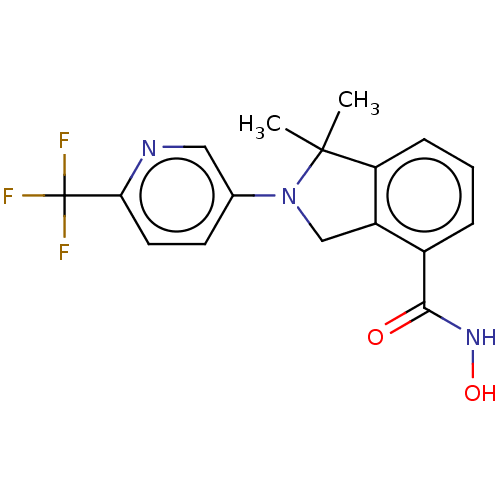 (CHEMBL4127735 | US11535607, Example 22-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50174298 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50174298 (3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273526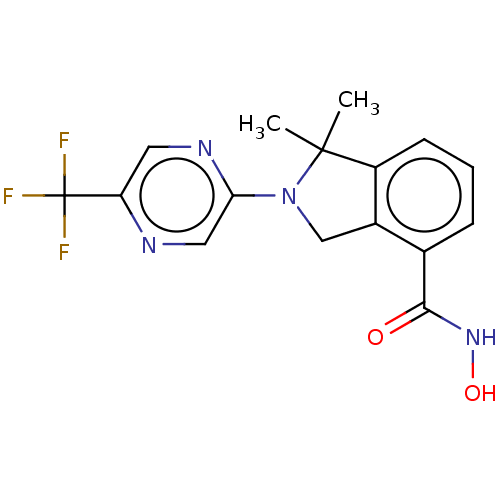 (CHEMBL4126661 | US11535607, Example 22-8) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421500 (US10508088, Example 1-1 | US10508088, ID HDTK010 |...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc. US Patent | Assay Description This example describes in vitro inhibition properties of exemplary HDAC11 inhibitors for various HDACs. HDAC inhibition assays were performed using a... | US Patent US10508088 (2019) BindingDB Entry DOI: 10.7270/Q2280B04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273553 (CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc. US Patent | Assay Description This example describes in vitro inhibition properties of exemplary HDAC11 inhibitors for various HDACs. HDAC inhibition assays were performed using a... | US Patent US10508088 (2019) BindingDB Entry DOI: 10.7270/Q2280B04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273524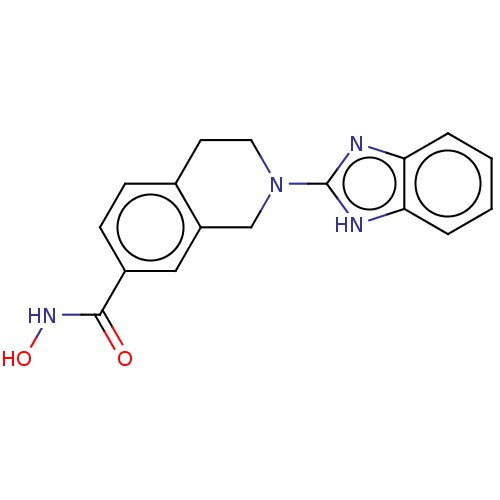 (CHEMBL4129402 | US11535607, Example 50-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50435691 (CHEMBL2391968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273538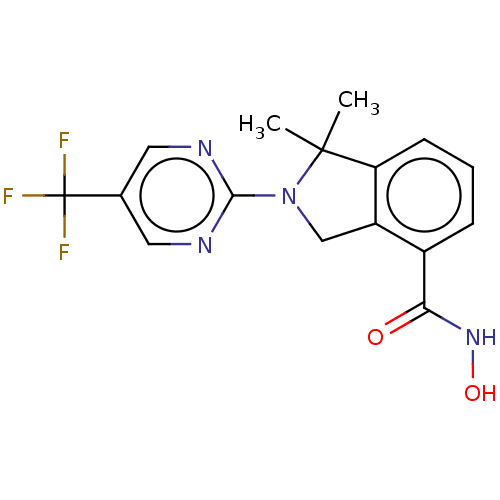 (CHEMBL4127894 | US11535607, Example 22-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340378 (3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273554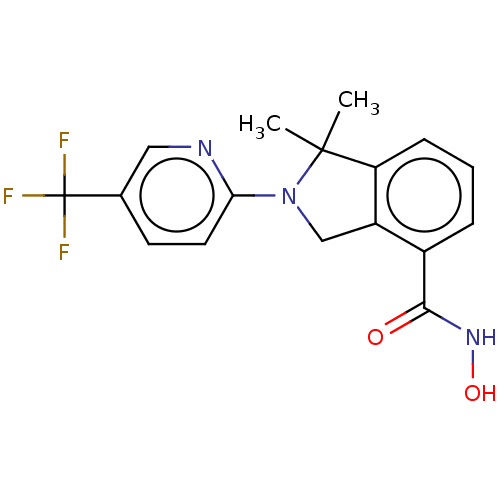 (CHEMBL4129134 | US11535607, Example 22-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273549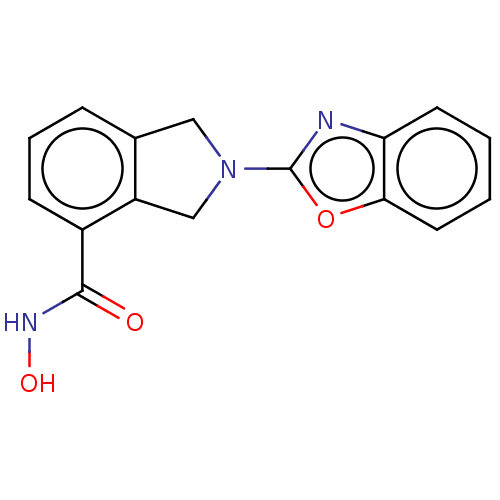 (CHEMBL4129266 | US11535607, Example 15-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273551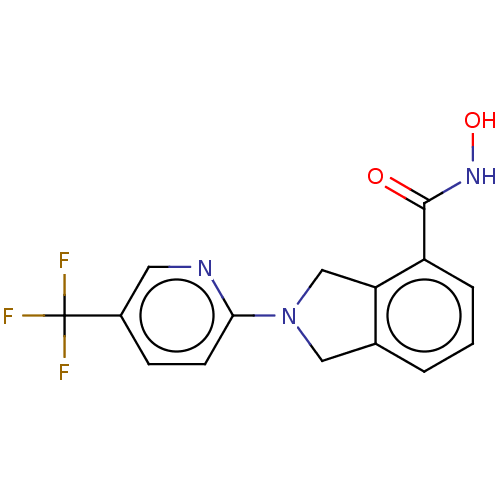 (CHEMBL4129143 | US11535607, Example 45-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340378 (3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50340386 (3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273520 (CHEMBL4127735 | US11535607, Example 22-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273525 (CHEMBL4128972 | US11535607, Example 18-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273523 (CHEMBL4129231 | US11535607, Example 7-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273536 (CHEMBL4126502 | US11535607, Example 22-7) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50435691 (CHEMBL2391968) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273521 (CHEMBL4129070 | US11535607, Example 50-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50340386 (3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 assessed as inhibition of conversion of radiolabeled cortisone to radiolabeled cortisol by cell-based assa... | Bioorg Med Chem Lett 23: 3650-3 (2013) Article DOI: 10.1016/j.bmcl.2013.03.011 BindingDB Entry DOI: 10.7270/Q29S1SDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273550 (CHEMBL4125954 | US11535607, Example 7-4) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273539 (CHEMBL4125815 | US11535607, Example 50-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273538 (CHEMBL4127894 | US11535607, Example 22-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273554 (CHEMBL4129134 | US11535607, Example 22-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273540 (CHEMBL4127193 | US11535607, Example 1-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273536 (CHEMBL4126502 | US11535607, Example 22-7) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273552 (CHEMBL4125788) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421722 (3-((6-(5-fluoro-2- (hydroxymethyl)phenyl)- 5-(trif...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421723 (N-hydroxy-3-((6-(3- (methoxymethyl)phenyl)-5- (tri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421724 (N-hydroxy-3-((6-(1-methyl-1H-indazol-6- yl)-5-(tri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421725 (3-(2-((3- (hydroxycarbamoyl)phenyl)amino)-5- (trif...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421726 (N-hydroxy-3-((6-(4-morpholinophenyl)-5- (trifluoro...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421727 (3-((6-(furan-3-yl)-5-(trifluoromethyl)-1H- benzo[d...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421728 (N-((benzylcarbamoyl)oxy)-3-((5-phenyl-6- (trifluor...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421730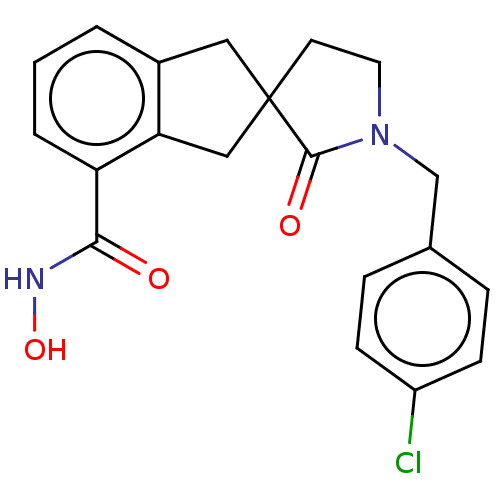 (1′-(4-chlorobenzyl)-N-hydroxy-2′-oxo-1...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM421732 ((R)-1′-(4-chloro-3-(trifluoromethyl)benzyl)-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM429898 (US10538496, Compound II-4) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM429899 (US10538496, Compound II-5) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics, Inc.; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description The probe binding HDAC11 assay was performed using a time resolved fluorescence (TRF) assay format. Recombinant N-terminal GST tag full-length human ... | US Patent US10538496 (2020) BindingDB Entry DOI: 10.7270/Q24J0HJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 478 total ) | Next | Last >> |