 Found 1022 hits with Last Name = 'hebach' and Initial = 'c'
Found 1022 hits with Last Name = 'hebach' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162854
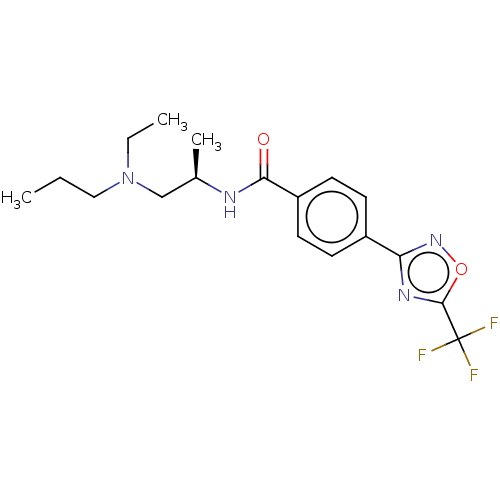
(US9056843, 145)Show SMILES CCCN(CC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C18H23F3N4O2/c1-4-10-25(5-2)11-12(3)22-16(26)14-8-6-13(7-9-14)15-23-17(27-24-15)18(19,20)21/h6-9,12H,4-5,10-11H2,1-3H3,(H,22,26)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162865
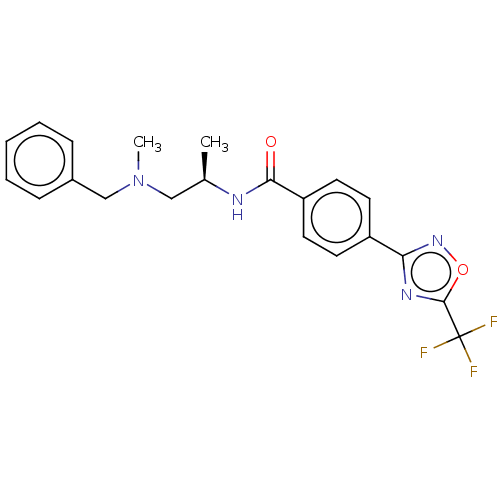
(US9056843, 156)Show SMILES C[C@H](CN(C)Cc1ccccc1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C21H21F3N4O2/c1-14(12-28(2)13-15-6-4-3-5-7-15)25-19(29)17-10-8-16(9-11-17)18-26-20(30-27-18)21(22,23)24/h3-11,14H,12-13H2,1-2H3,(H,25,29)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162846

(US9056843, 137)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-26(11-5-2)12-13(3)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,4-5,10-12H2,1-3H3,(H,23,27)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162848

(US9056843, 139)Show SMILES CCCN(C)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H21F3N4O2/c1-4-9-24(3)10-11(2)21-15(25)13-7-5-12(6-8-13)14-22-16(26-23-14)17(18,19)20/h5-8,11H,4,9-10H2,1-3H3,(H,21,25)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162859

(US9056843, 150)Show SMILES CCCCN(C)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C18H23F3N4O2/c1-4-5-10-25(3)11-12(2)22-16(26)14-8-6-13(7-9-14)15-23-17(27-24-15)18(19,20)21/h6-9,12H,4-5,10-11H2,1-3H3,(H,22,26)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162852
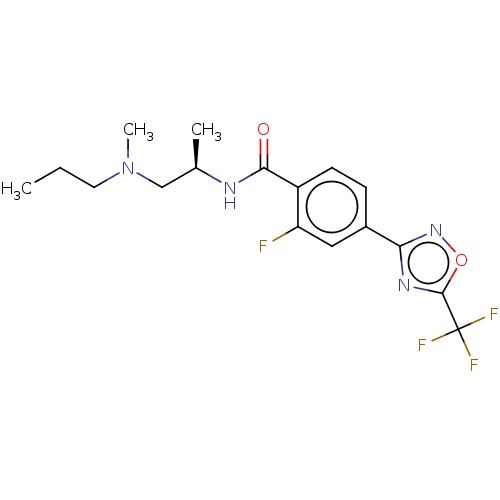
(US9056843, 143)Show SMILES CCCN(C)C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H20F4N4O2/c1-4-7-25(3)9-10(2)22-15(26)12-6-5-11(8-13(12)18)14-23-16(27-24-14)17(19,20)21/h5-6,8,10H,4,7,9H2,1-3H3,(H,22,26)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162850
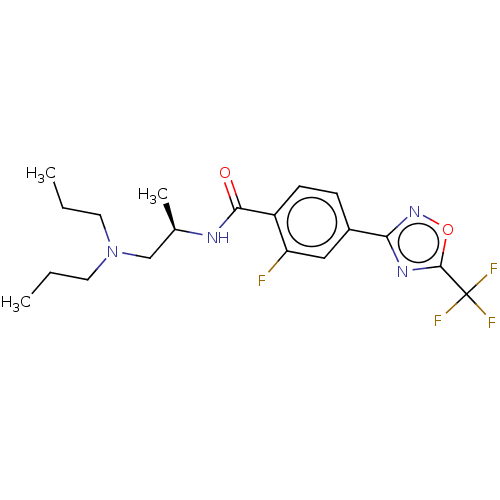
(US9056843, 141)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H24F4N4O2/c1-4-8-27(9-5-2)11-12(3)24-17(28)14-7-6-13(10-15(14)20)16-25-18(29-26-16)19(21,22)23/h6-7,10,12H,4-5,8-9,11H2,1-3H3,(H,24,28)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162855
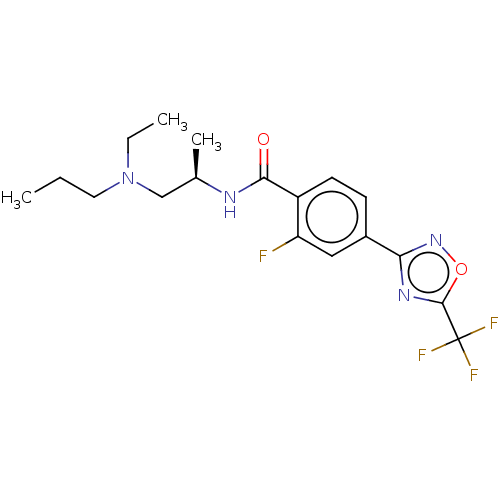
(US9056843, 146)Show SMILES CCCN(CC)C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C18H22F4N4O2/c1-4-8-26(5-2)10-11(3)23-16(27)13-7-6-12(9-14(13)19)15-24-17(28-25-15)18(20,21)22/h6-7,9,11H,4-5,8,10H2,1-3H3,(H,23,27)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162860
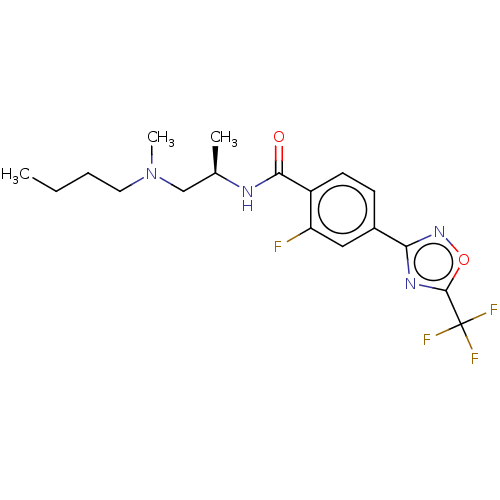
(US9056843, 151)Show SMILES CCCCN(C)C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C18H22F4N4O2/c1-4-5-8-26(3)10-11(2)23-16(27)13-7-6-12(9-14(13)19)15-24-17(28-25-15)18(20,21)22/h6-7,9,11H,4-5,8,10H2,1-3H3,(H,23,27)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533774

(CHEMBL4469006)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(c1)-c1ncnc2ccc(cc12)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H25N5O2/c1-20(36)34-11-13-35(14-12-34)30(37)24-7-4-6-23(16-24)29-26-17-21(9-10-28(26)32-19-33-29)25-15-22-5-2-3-8-27(22)31-18-25/h2-10,15-19H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118301
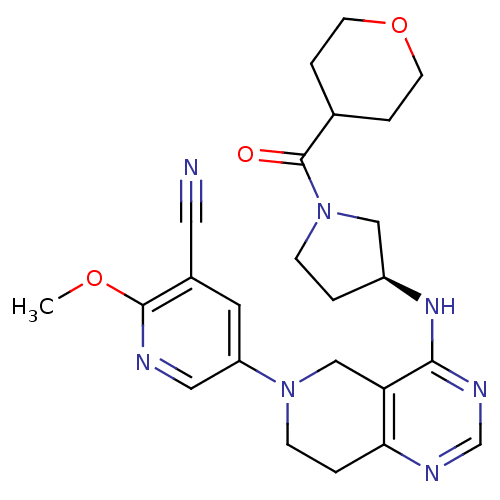
(US8653092, 69)Show SMILES COc1ncc(cc1C#N)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C24H29N7O3/c1-33-23-17(11-25)10-19(12-26-23)30-7-3-21-20(14-30)22(28-15-27-21)29-18-2-6-31(13-18)24(32)16-4-8-34-9-5-16/h10,12,15-16,18H,2-9,13-14H2,1H3,(H,27,28,29)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118303

(US8653092, 71)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOC(C)(C)C3)c2C1 |r| Show InChI InChI=1S/C26H33F3N6O3/c1-25(2)11-16(6-9-38-25)24(36)35-7-4-17(13-35)33-22-19-14-34(8-5-21(19)31-15-32-22)18-10-20(26(27,28)29)23(37-3)30-12-18/h10,12,15-17H,4-9,11,13-14H2,1-3H3,(H,31,32,33)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118314
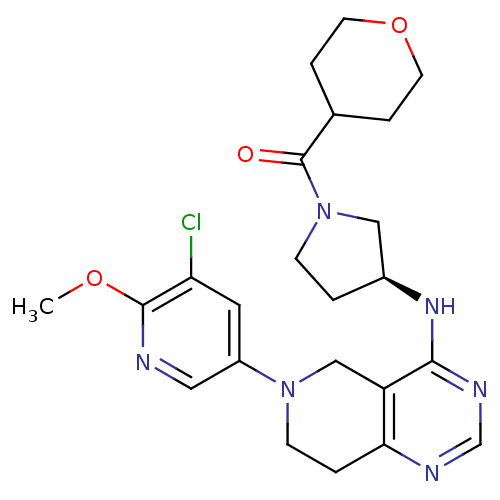
(US8653092, 82)Show SMILES COc1ncc(cc1Cl)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C23H29ClN6O3/c1-32-22-19(24)10-17(11-25-22)29-7-3-20-18(13-29)21(27-14-26-20)28-16-2-6-30(12-16)23(31)15-4-8-33-9-5-15/h10-11,14-16H,2-9,12-13H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162862
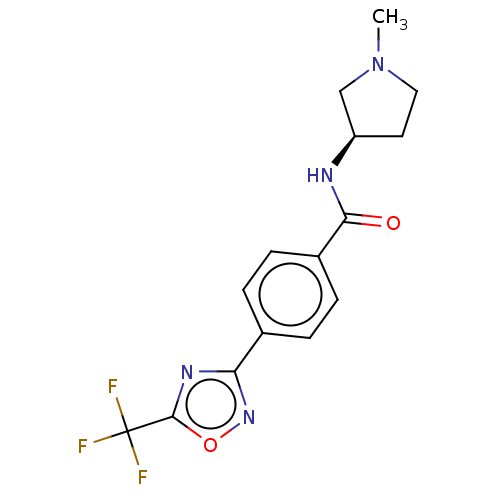
(US9056843, 153)Show SMILES CN1CC[C@H](C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-22-7-6-11(8-22)19-13(23)10-4-2-9(3-5-10)12-20-14(24-21-12)15(16,17)18/h2-5,11H,6-8H2,1H3,(H,19,23)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118305
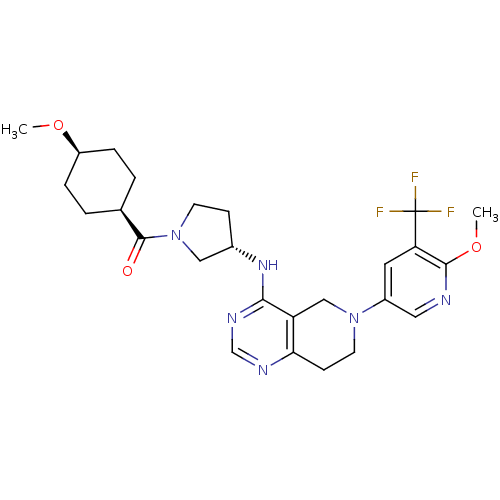
(US8653092, 73)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)N1CC[C@@H](C1)Nc1ncnc2CCN(Cc12)c1cnc(OC)c(c1)C(F)(F)F |r,wU:5.8,2.1,wD:13.16,(10.58,2.37,;9.81,3.7,;8.27,3.7,;7.5,2.37,;5.96,2.37,;5.19,3.7,;5.96,5.04,;7.5,5.04,;3.65,3.7,;2.88,5.04,;2.88,2.37,;3.79,1.12,;2.88,-.12,;1.42,.35,;1.42,1.89,;.09,-.42,;.09,-1.96,;1.42,-2.73,;1.42,-4.27,;.09,-5.04,;-1.25,-4.27,;-2.58,-5.04,;-3.92,-4.27,;-3.92,-2.73,;-2.58,-1.96,;-1.25,-2.73,;-5.25,-1.96,;-5.25,-.42,;-6.58,.35,;-7.92,-.42,;-9.25,.35,;-9.25,1.89,;-7.92,-1.96,;-6.58,-2.73,;-9.25,-2.73,;-10.58,-1.96,;-9.25,-4.27,;-10.02,-1.39,)| Show InChI InChI=1S/C26H33F3N6O3/c1-37-19-5-3-16(4-6-19)25(36)35-9-7-17(13-35)33-23-20-14-34(10-8-22(20)31-15-32-23)18-11-21(26(27,28)29)24(38-2)30-12-18/h11-12,15-17,19H,3-10,13-14H2,1-2H3,(H,31,32,33)/t16-,17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118261

(US8653092, 28)Show SMILES COc1ncc(cc1C#N)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3oc(C)nc3C)c2C1 |r| Show InChI InChI=1S/C24H25N7O4/c1-14-21(34-15(2)29-14)24(32)31-6-4-18(11-31)35-23-19-12-30(7-5-20(19)27-13-28-23)17-8-16(9-25)22(33-3)26-10-17/h8,10,13,18H,4-7,11-12H2,1-3H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118307

(US8653092, 75)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)[C@@H]3CC[C@H](O)CC3)c2C1 |r,wU:28.29,31.33,wD:21.21,(-8.87,1.89,;-8.87,.35,;-7.53,-.42,;-6.2,.35,;-4.86,-.42,;-4.86,-1.96,;-6.2,-2.73,;-7.53,-1.96,;-8.87,-2.73,;-10.2,-1.96,;-8.87,-4.27,;-9.64,-1.39,;-3.53,-2.73,;-3.53,-4.27,;-2.2,-5.04,;-.86,-4.27,;.47,-5.04,;1.8,-4.27,;1.8,-2.73,;.47,-1.96,;.47,-.42,;1.8,.35,;3.27,-.12,;4.17,1.12,;3.27,2.37,;1.8,1.89,;4.04,3.7,;3.27,5.04,;5.58,3.7,;6.35,2.37,;7.89,2.37,;8.66,3.7,;10.2,3.7,;7.89,5.04,;6.35,5.04,;-.86,-2.73,;-2.2,-1.96,)| Show InChI InChI=1S/C25H31F3N6O3/c1-37-23-20(25(26,27)28)10-17(11-29-23)33-9-7-21-19(13-33)22(31-14-30-21)32-16-6-8-34(12-16)24(36)15-2-4-18(35)5-3-15/h10-11,14-16,18,35H,2-9,12-13H2,1H3,(H,30,31,32)/t15-,16-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118304

(US8653092, 72)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)c3cnco3)c2C1 |r| Show InChI InChI=1S/C22H22F3N7O3/c1-34-20-16(22(23,24)25)6-14(7-27-20)31-5-3-17-15(10-31)19(29-11-28-17)30-13-2-4-32(9-13)21(33)18-8-26-12-35-18/h6-8,11-13H,2-5,9-10H2,1H3,(H,28,29,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118306

(US8653092, 74)Show SMILES CO[C@H]1CC[C@@H](CC1)C(=O)N1CC[C@@H](C1)Nc1ncnc2CCN(Cc12)c1cnc(OC)c(c1)C(F)(F)F |r,wU:5.8,wD:13.16,2.1,(10.58,2.37,;9.81,3.7,;8.27,3.7,;7.5,5.04,;5.96,5.04,;5.19,3.7,;5.96,2.37,;7.5,2.37,;3.65,3.7,;2.88,5.04,;2.88,2.37,;3.79,1.12,;2.88,-.12,;1.42,.35,;1.42,1.89,;.09,-.42,;.09,-1.96,;1.42,-2.73,;1.42,-4.27,;.09,-5.04,;-1.25,-4.27,;-2.58,-5.04,;-3.92,-4.27,;-3.92,-2.73,;-2.58,-1.96,;-1.25,-2.73,;-5.25,-1.96,;-5.25,-.42,;-6.58,.35,;-7.92,-.42,;-9.25,.35,;-9.25,1.89,;-7.92,-1.96,;-6.58,-2.73,;-9.25,-2.73,;-10.58,-1.96,;-9.25,-4.27,;-10.02,-1.39,)| Show InChI InChI=1S/C26H33F3N6O3/c1-37-19-5-3-16(4-6-19)25(36)35-9-7-17(13-35)33-23-20-14-34(10-8-22(20)31-15-32-23)18-11-21(26(27,28)29)24(38-2)30-12-18/h11-12,15-17,19H,3-10,13-14H2,1-2H3,(H,31,32,33)/t16-,17-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162847
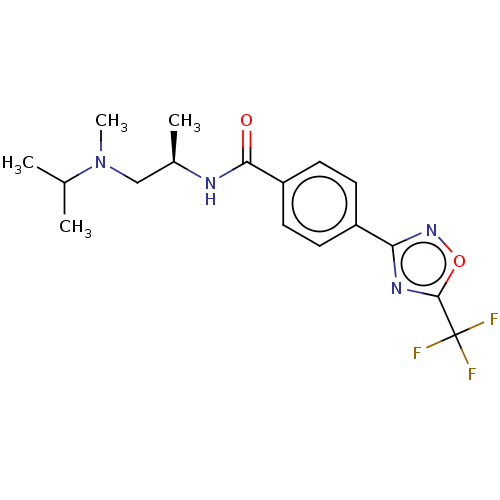
(US9056843, 138)Show SMILES C[C@H](CN(C)C(C)C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H21F3N4O2/c1-10(2)24(4)9-11(3)21-15(25)13-7-5-12(6-8-13)14-22-16(26-23-14)17(18,19)20/h5-8,10-11H,9H2,1-4H3,(H,21,25)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203680
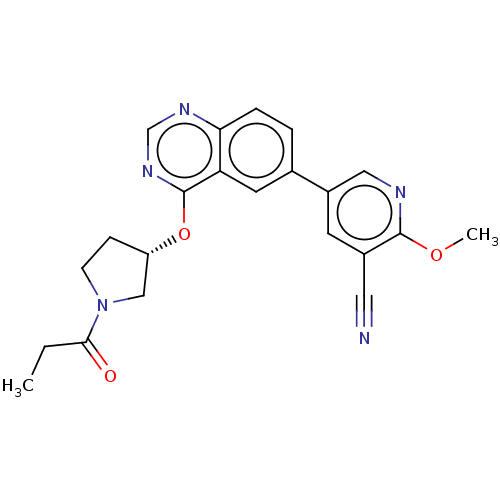
(CHEMBL3960012)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1cnc(OC)c(c1)C#N |r| Show InChI InChI=1S/C22H21N5O3/c1-3-20(28)27-7-6-17(12-27)30-22-18-9-14(4-5-19(18)25-13-26-22)16-8-15(10-23)21(29-2)24-11-16/h4-5,8-9,11,13,17H,3,6-7,12H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533772

(CHEMBL4521888)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24F3N5O3/c1-17(37)35-8-10-36(11-9-35)27(38)20-5-3-4-19(12-20)25-22-13-18(6-7-24(22)33-16-34-25)21-14-23(28(29,30)31)26(39-2)32-15-21/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118287
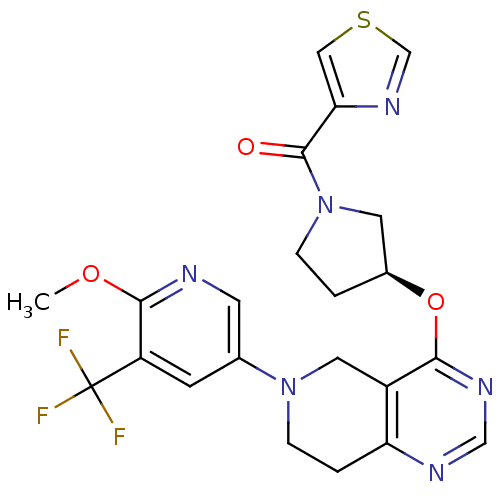
(US8653092, 55)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3cscn3)c2C1 |r| Show InChI InChI=1S/C22H21F3N6O3S/c1-33-20-16(22(23,24)25)6-13(7-26-20)30-5-3-17-15(9-30)19(28-11-27-17)34-14-2-4-31(8-14)21(32)18-10-35-12-29-18/h6-7,10-12,14H,2-5,8-9H2,1H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162841

(US9056843, 132)Show SMILES CCN(CC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H21F3N4O2/c1-4-24(5-2)10-11(3)21-15(25)13-8-6-12(7-9-13)14-22-16(26-23-14)17(18,19)20/h6-9,11H,4-5,10H2,1-3H3,(H,21,25)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118286
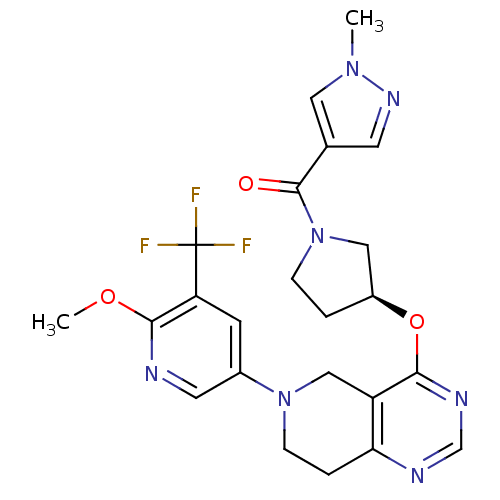
(US8653092, 54)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3cnn(C)c3)c2C1 |r| Show InChI InChI=1S/C23H24F3N7O3/c1-31-10-14(8-30-31)22(34)33-5-3-16(11-33)36-20-17-12-32(6-4-19(17)28-13-29-20)15-7-18(23(24,25)26)21(35-2)27-9-15/h7-10,13,16H,3-6,11-12H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR after 60 mins in presence of [gamma-33P]-ATP by microplate scintillation counting |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118273
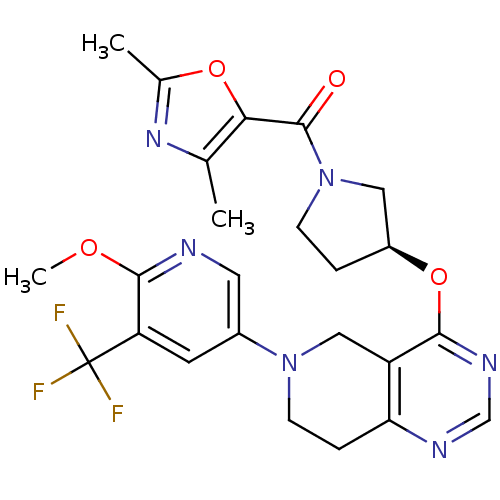
(US8653092, 40)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3oc(C)nc3C)c2C1 |r| Show InChI InChI=1S/C24H25F3N6O4/c1-13-20(36-14(2)31-13)23(34)33-6-4-16(10-33)37-21-17-11-32(7-5-19(17)29-12-30-21)15-8-18(24(25,26)27)22(35-3)28-9-15/h8-9,12,16H,4-7,10-11H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118302

(US8653092, 70)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCN(CC3)C(C)=O)c2C1 |r| Show InChI InChI=1S/C26H32F3N7O3/c1-16(37)34-7-3-17(4-8-34)25(38)36-9-5-18(13-36)33-23-20-14-35(10-6-22(20)31-15-32-23)19-11-21(26(27,28)29)24(39-2)30-12-19/h11-12,15,17-18H,3-10,13-14H2,1-2H3,(H,31,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118285
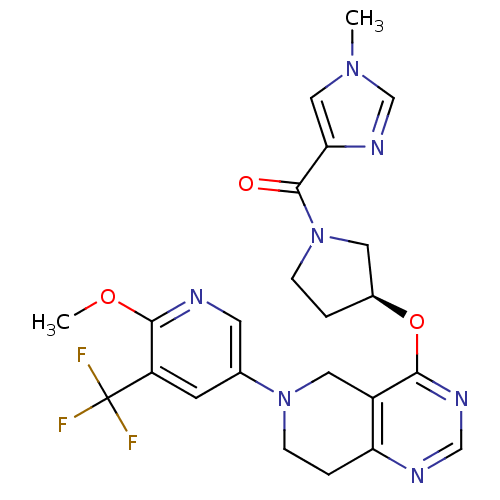
(US8653092, 53)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3cn(C)cn3)c2C1 |r| Show InChI InChI=1S/C23H24F3N7O3/c1-31-11-19(30-13-31)22(34)33-5-3-15(9-33)36-20-16-10-32(6-4-18(16)28-12-29-20)14-7-17(23(24,25)26)21(35-2)27-8-14/h7-8,11-13,15H,3-6,9-10H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [2-1084]
(Homo sapiens (Human)) | BDBM187784

(US9670193, 36 3-chloro-N-(1-(pyridin-4-yl)ethyl)-5...)Show SMILES CC(Nc1ncc(cc1Cl)-c1noc(n1)C(F)(F)F)c1ccncc1 Show InChI InChI=1S/C15H11ClF3N5O/c1-8(9-2-4-20-5-3-9)22-13-11(16)6-10(7-21-13)12-23-14(25-24-12)15(17,18)19/h2-8H,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Novartis AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9670193 (2017)
BindingDB Entry DOI: 10.7270/Q2SF2TCV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118308
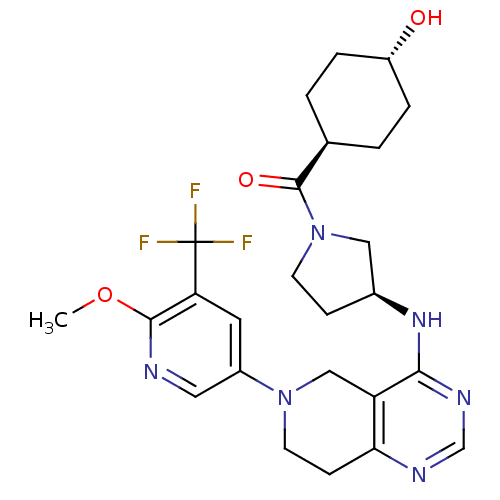
(US8653092, 76)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)[C@H]3CC[C@H](O)CC3)c2C1 |r,wU:28.29,wD:21.21,31.33,(-8.87,1.89,;-8.87,.35,;-7.53,-.42,;-6.2,.35,;-4.86,-.42,;-4.86,-1.96,;-6.2,-2.73,;-7.53,-1.96,;-8.87,-2.73,;-10.2,-1.96,;-8.87,-4.27,;-9.64,-1.39,;-3.53,-2.73,;-3.53,-4.27,;-2.2,-5.04,;-.86,-4.27,;.47,-5.04,;1.8,-4.27,;1.8,-2.73,;.47,-1.96,;.47,-.42,;1.8,.35,;3.27,-.12,;4.17,1.12,;3.27,2.37,;1.8,1.89,;4.04,3.7,;3.27,5.04,;5.58,3.7,;6.35,5.04,;7.89,5.04,;8.66,3.7,;10.2,3.7,;7.89,2.37,;6.35,2.37,;-.86,-2.73,;-2.2,-1.96,)| Show InChI InChI=1S/C25H31F3N6O3/c1-37-23-20(25(26,27)28)10-17(11-29-23)33-9-7-21-19(13-33)22(31-14-30-21)32-16-6-8-34(12-16)24(36)15-2-4-18(35)5-3-15/h10-11,14-16,18,35H,2-9,12-13H2,1H3,(H,30,31,32)/t15-,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699
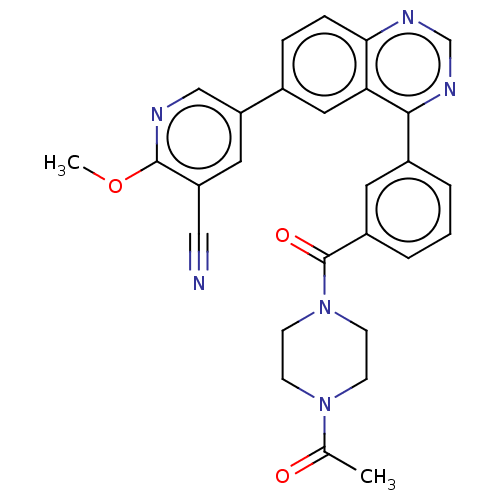
(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118300

(US8653092, 68)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C24H29F3N6O3/c1-35-22-19(24(25,26)27)10-17(11-28-22)32-7-3-20-18(13-32)21(30-14-29-20)31-16-2-6-33(12-16)23(34)15-4-8-36-9-5-15/h10-11,14-16H,2-9,12-13H2,1H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162851
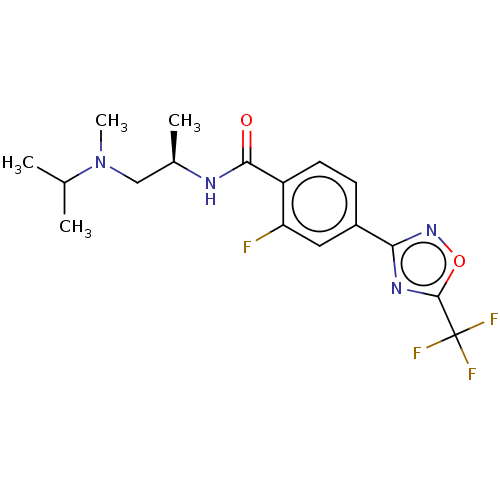
(US9056843, 142)Show SMILES C[C@H](CN(C)C(C)C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H20F4N4O2/c1-9(2)25(4)8-10(3)22-15(26)12-6-5-11(7-13(12)18)14-23-16(27-24-14)17(19,20)21/h5-7,9-10H,8H2,1-4H3,(H,22,26)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162832

(US9056843, 123)Show SMILES CCN(CC)CC(C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F Show InChI InChI=1S/C17H21F3N4O2/c1-4-24(5-2)10-11(3)21-15(25)13-8-6-12(7-9-13)14-22-16(26-23-14)17(18,19)20/h6-9,11H,4-5,10H2,1-3H3,(H,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118254

(US8653092, 21)Show SMILES COc1cc(cnc1OC)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3cocn3)c2C1 |r| Show InChI InChI=1S/C22H24N6O5/c1-30-19-7-14(8-23-21(19)31-2)27-6-4-17-16(10-27)20(25-12-24-17)33-15-3-5-28(9-15)22(29)18-11-32-13-26-18/h7-8,11-13,15H,3-6,9-10H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118255
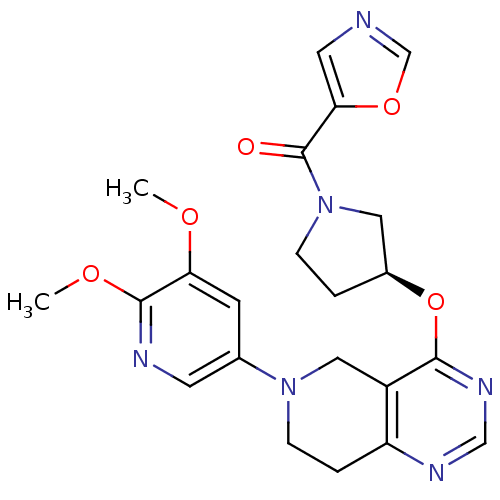
(US8653092, 22)Show SMILES COc1cc(cnc1OC)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3cnco3)c2C1 |r| Show InChI InChI=1S/C22H24N6O5/c1-30-18-7-14(8-24-21(18)31-2)27-6-4-17-16(11-27)20(26-12-25-17)33-15-3-5-28(10-15)22(29)19-9-23-13-32-19/h7-9,12-13,15H,3-6,10-11H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162861
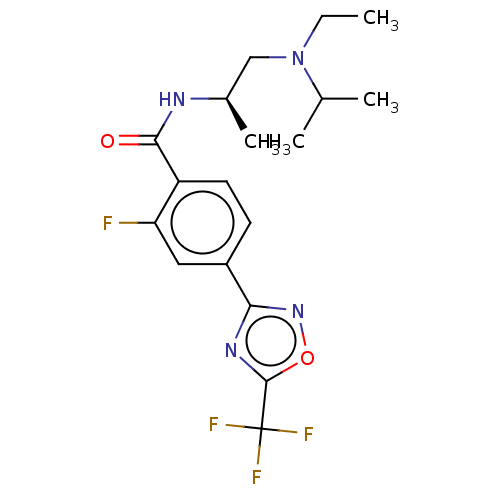
(US9056843, 152)Show SMILES CCN(C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C18H22F4N4O2/c1-5-26(10(2)3)9-11(4)23-16(27)13-7-6-12(8-14(13)19)15-24-17(28-25-15)18(20,21)22/h6-8,10-11H,5,9H2,1-4H3,(H,23,27)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162835
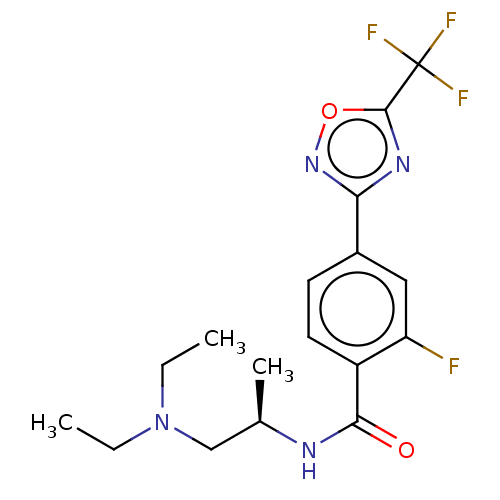
(US9056843, 126)Show SMILES CCN(CC)C[C@@H](C)NC(=O)c1ccc(cc1F)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H20F4N4O2/c1-4-25(5-2)9-10(3)22-15(26)12-7-6-11(8-13(12)18)14-23-16(27-24-14)17(19,20)21/h6-8,10H,4-5,9H2,1-3H3,(H,22,26)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162845

(US9056843, 136)Show SMILES CCN(C)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C16H19F3N4O2/c1-4-23(3)9-10(2)20-14(24)12-7-5-11(6-8-12)13-21-15(25-22-13)16(17,18)19/h5-8,10H,4,9H2,1-3H3,(H,20,24)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... |
US Patent US9056843 (2015)
BindingDB Entry DOI: 10.7270/Q24F1PG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203701
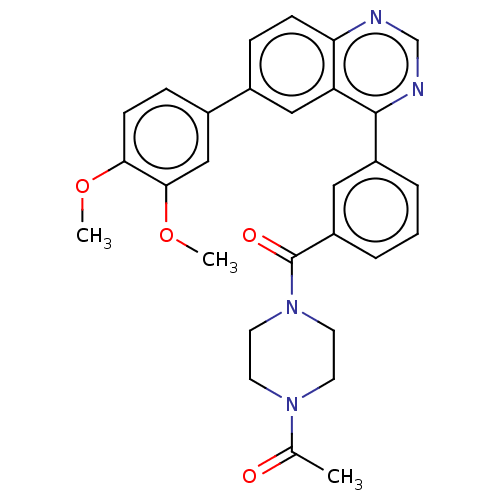
(CHEMBL3944013)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C29H28N4O4/c1-19(34)32-11-13-33(14-12-32)29(35)23-6-4-5-22(15-23)28-24-16-20(7-9-25(24)30-18-31-28)21-8-10-26(36-2)27(17-21)37-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699

(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533778
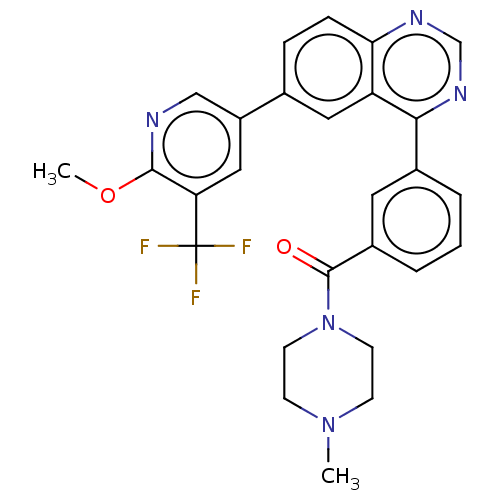
(CHEMBL4453497)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C27H24F3N5O2/c1-34-8-10-35(11-9-34)26(36)19-5-3-4-18(12-19)24-21-13-17(6-7-23(21)32-16-33-24)20-14-22(27(28,29)30)25(37-2)31-15-20/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118316
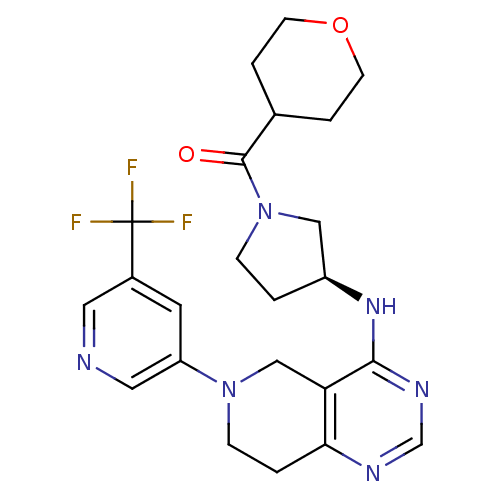
(US8653092, 84)Show SMILES FC(F)(F)c1cncc(c1)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C23H27F3N6O2/c24-23(25,26)16-9-18(11-27-10-16)31-6-2-20-19(13-31)21(29-14-28-20)30-17-1-5-32(12-17)22(33)15-3-7-34-8-4-15/h9-11,14-15,17H,1-8,12-13H2,(H,28,29,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203696
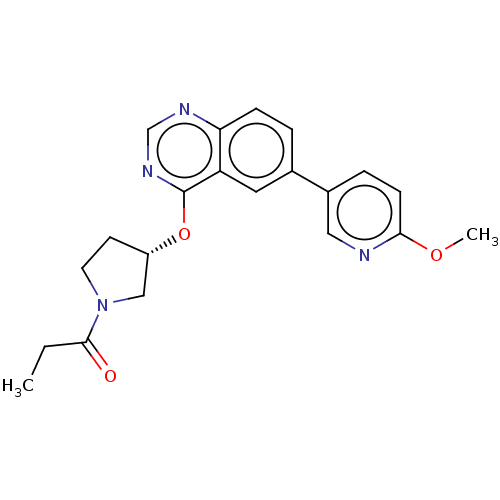
(CHEMBL3896413)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H22N4O3/c1-3-20(26)25-9-8-16(12-25)28-21-17-10-14(4-6-18(17)23-13-24-21)15-5-7-19(27-2)22-11-15/h4-7,10-11,13,16H,3,8-9,12H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118272
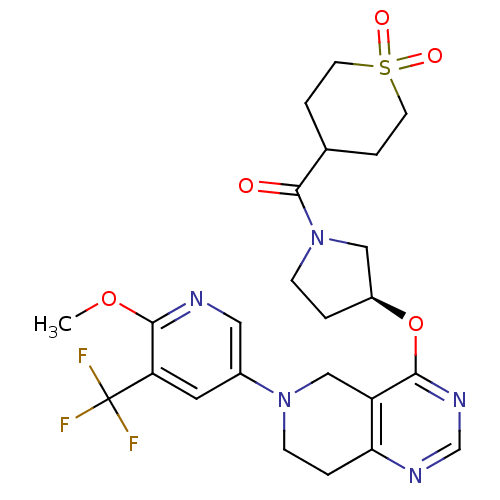
(US8653092, 39)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)c2C1 |r| Show InChI InChI=1S/C24H28F3N5O5S/c1-36-22-19(24(25,26)27)10-16(11-28-22)31-7-3-20-18(13-31)21(30-14-29-20)37-17-2-6-32(12-17)23(33)15-4-8-38(34,35)9-5-15/h10-11,14-15,17H,2-9,12-13H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118235

(US8653092, 2)Show SMILES COc1ncc(N2CCc3ncnc(O[C@H]4CCN(C4)C(=O)C4CCOCC4)c3C2)c(OC)n1 |r| Show InChI InChI=1S/C23H30N6O5/c1-31-21-19(11-24-23(27-21)32-2)28-8-4-18-17(13-28)20(26-14-25-18)34-16-3-7-29(12-16)22(30)15-5-9-33-10-6-15/h11,14-16H,3-10,12-13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118242
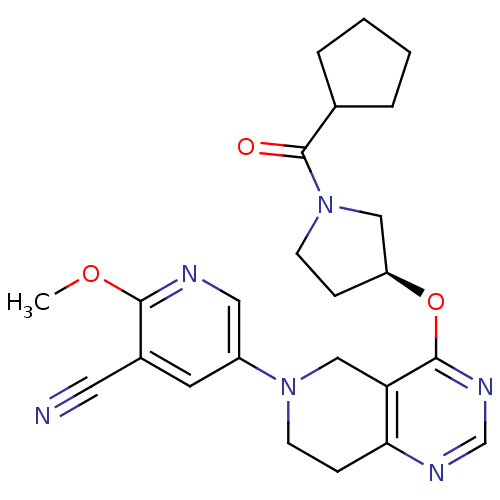
(US8653092, 9)Show SMILES COc1ncc(cc1C#N)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)C3CCCC3)c2C1 |r| Show InChI InChI=1S/C24H28N6O3/c1-32-22-17(11-25)10-18(12-26-22)29-9-7-21-20(14-29)23(28-15-27-21)33-19-6-8-30(13-19)24(31)16-4-2-3-5-16/h10,12,15-16,19H,2-9,13-14H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118243

(US8653092, 10)Show SMILES COc1ncc(cc1C)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)c3oc(C)nc3C)c2C1 |r| Show InChI InChI=1S/C24H28N6O4/c1-14-9-17(10-25-22(14)32-4)29-8-6-20-19(12-29)23(27-13-26-20)34-18-5-7-30(11-18)24(31)21-15(2)28-16(3)33-21/h9-10,13,18H,5-8,11-12H2,1-4H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118257

(US8653092, 24)Show SMILES COc1cc(cnc1OC)N1CCc2ncnc(O[C@H]3CCN(C3)C(=O)C3CCOC(C)(C)C3)c2C1 |r| Show InChI InChI=1S/C26H35N5O5/c1-26(2)12-17(7-10-35-26)25(32)31-8-5-19(14-31)36-23-20-15-30(9-6-21(20)28-16-29-23)18-11-22(33-3)24(34-4)27-13-18/h11,13,16-17,19H,5-10,12,14-15H2,1-4H3/t17?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi... |
US Patent US8653092 (2014)
BindingDB Entry DOI: 10.7270/Q2M61HXZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data