

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apelin receptor (Homo sapiens (Human)) | BDBM50588316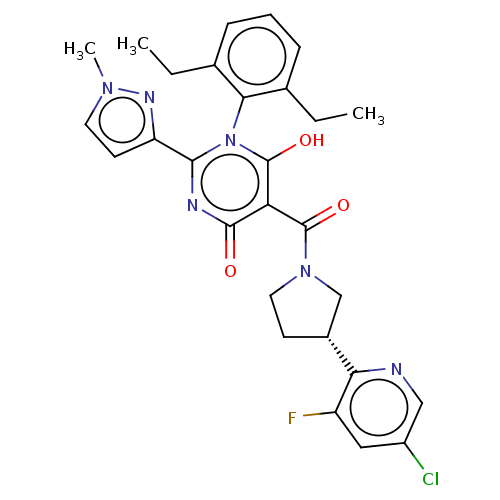 (CHEMBL5170657) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01504 BindingDB Entry DOI: 10.7270/Q21V5JX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50567169 (CHEMBL4873876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50014619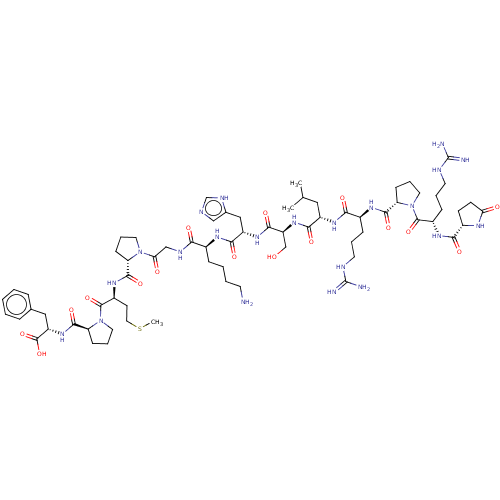 (CHEMBL3184840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50554439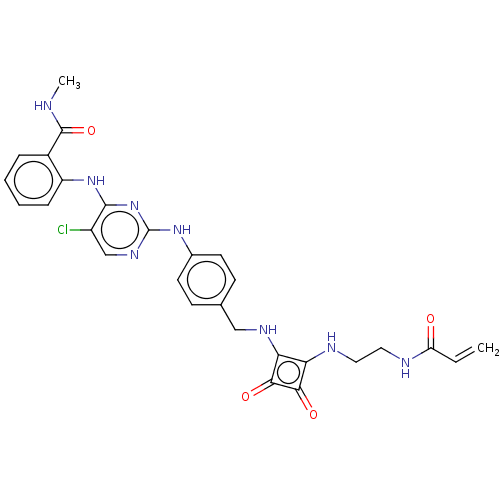 (CHEMBL4752642) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Pol... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01059 BindingDB Entry DOI: 10.7270/Q2F193CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18790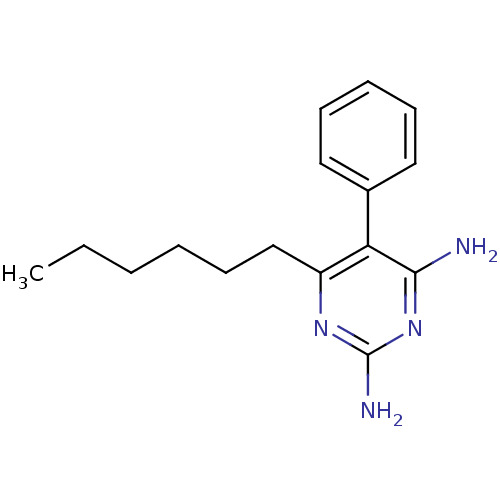 (6-hexyl-5-phenylpyrimidine-2,4-diamine | CHEMBL416...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18778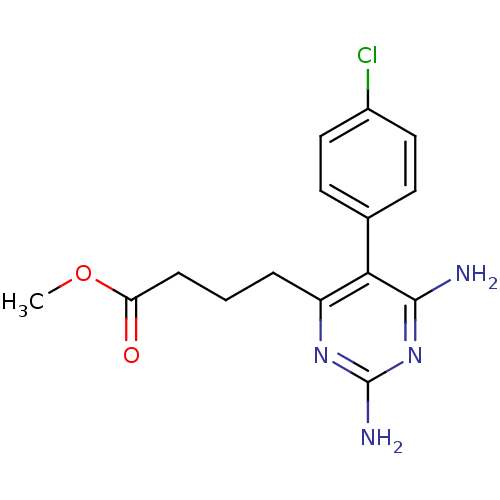 (CHEMBL22405 | P16 | methyl 4-[2,6-diamino-5-(4-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18781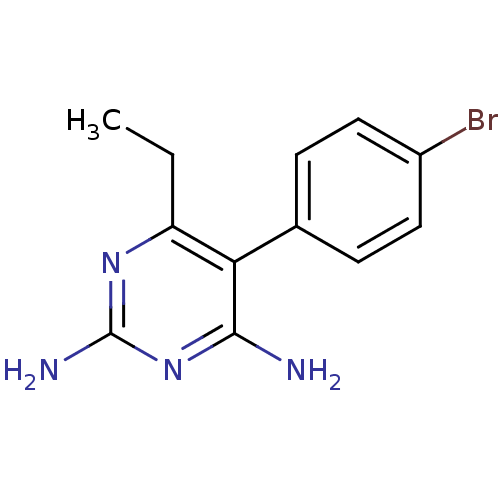 (5-(4-bromophenyl)-6-ethylpyrimidine-2,4-diamine | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110753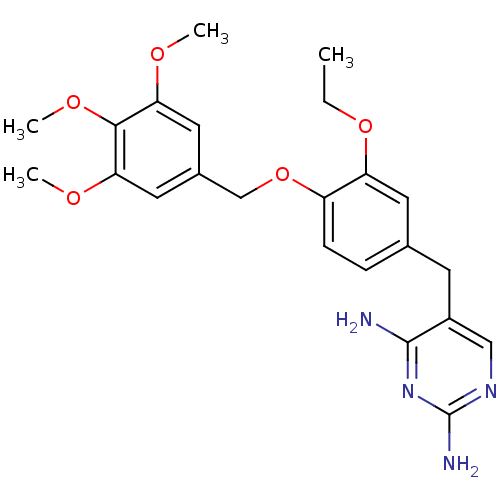 (5-(3-ethoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110753 (5-(3-ethoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110755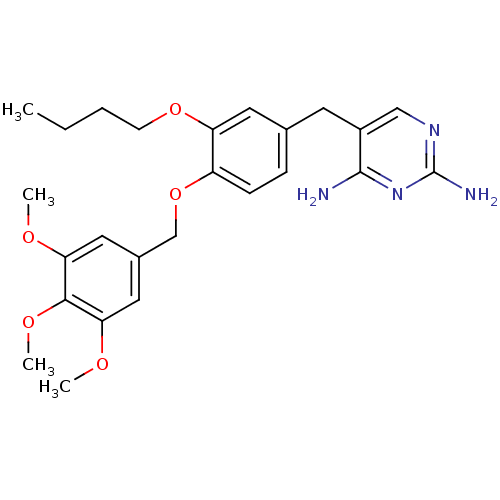 (5-(3-butoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110769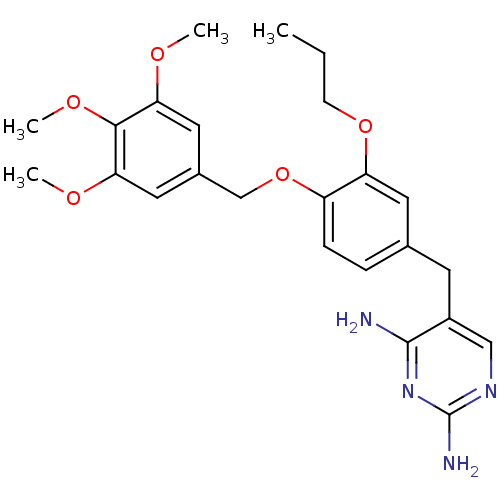 (5-(3-propoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110769 (5-(3-propoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787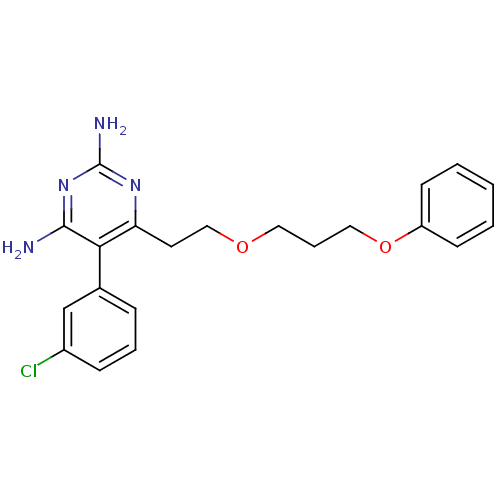 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18779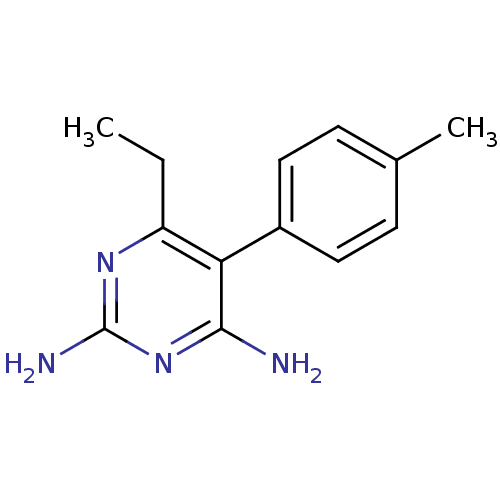 (6-ethyl-5-(4-methylphenyl)pyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110755 (5-(3-butoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50554449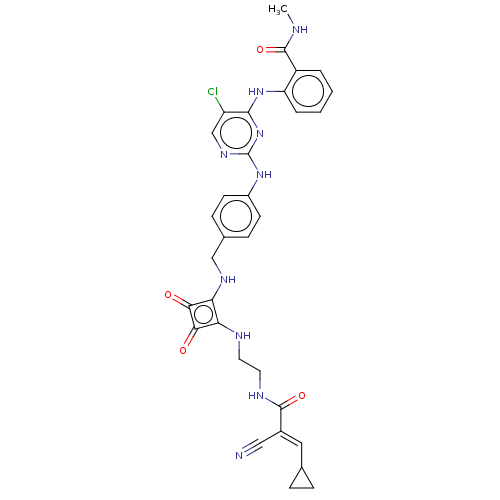 (CHEMBL4750273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Pol... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01059 BindingDB Entry DOI: 10.7270/Q2F193CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50554448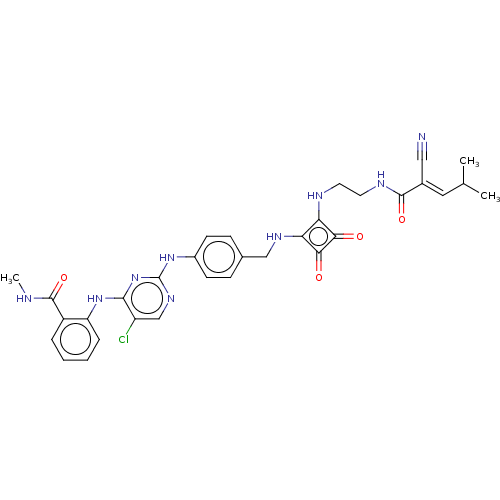 (CHEMBL4783568) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human N-terminal His-tagged FAK (393 to 698 residues) expressed in baculovirus infected Sf9 insect cells using Pol... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01059 BindingDB Entry DOI: 10.7270/Q2F193CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18791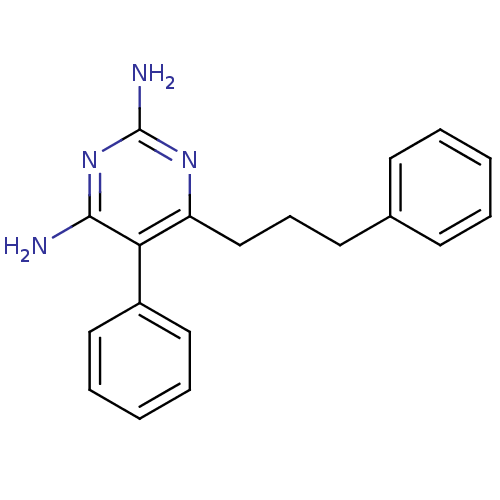 (5-phenyl-6-(3-phenylpropyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110767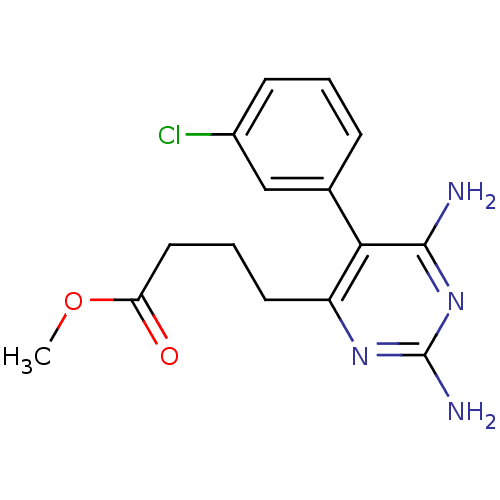 (4-[2,6-Diamino-5-(3-chloro-phenyl)-pyrimidin-4-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110760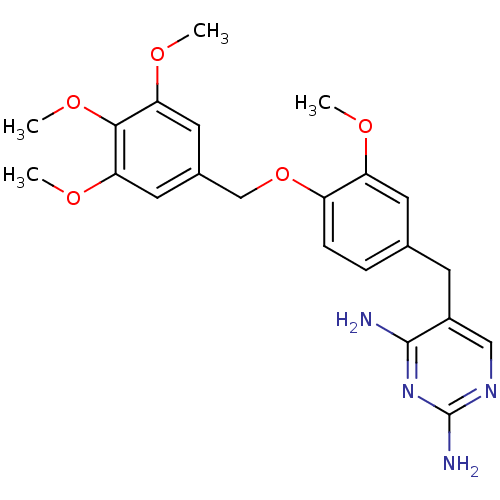 (5-(3-methoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138705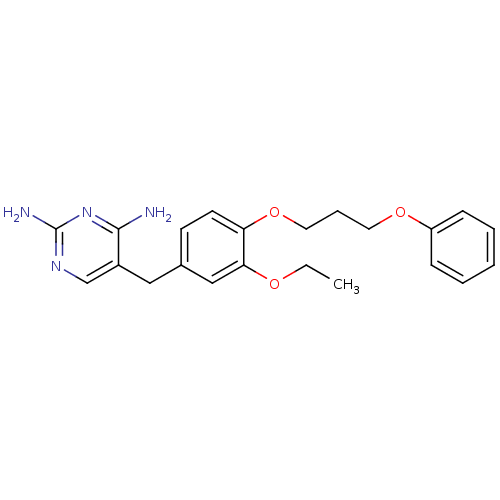 (5-[3-Ethoxy-4-(3-phenoxy-propoxy)-benzyl]-pyrimidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110759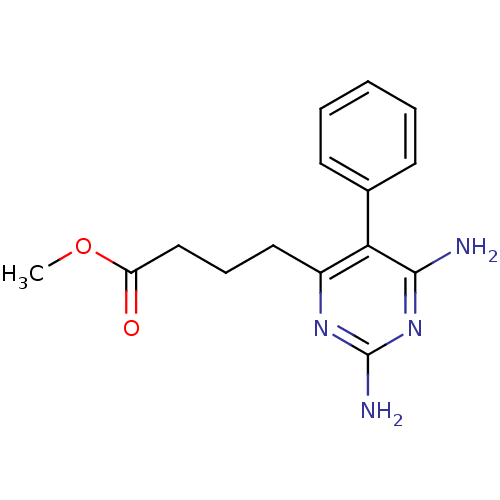 (4-(2,6-Diamino-5-phenyl-pyrimidin-4-yl)-butyric ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110760 (5-(3-methoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18782 (5-(4-tert-butylphenyl)-6-ethylpyrimidine-2,4-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18790 (6-hexyl-5-phenylpyrimidine-2,4-diamine | CHEMBL416...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the C59R+S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110772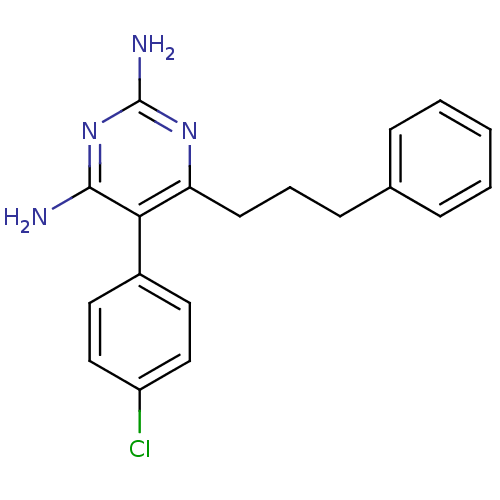 (5-(4-Chloro-phenyl)-6-(3-phenyl-propyl)-pyrimidine...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110758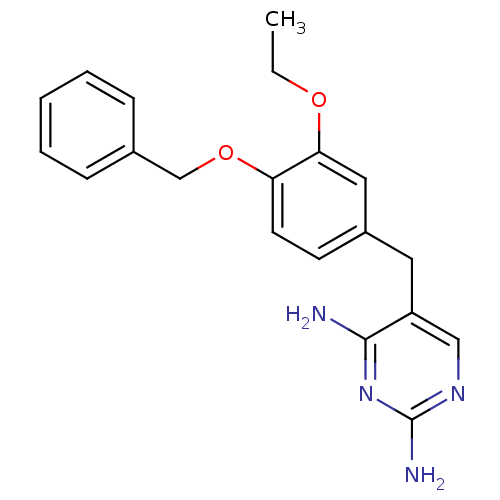 (5-(4-(benzyloxy)-3-ethoxybenzyl)pyrimidine-2,4-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784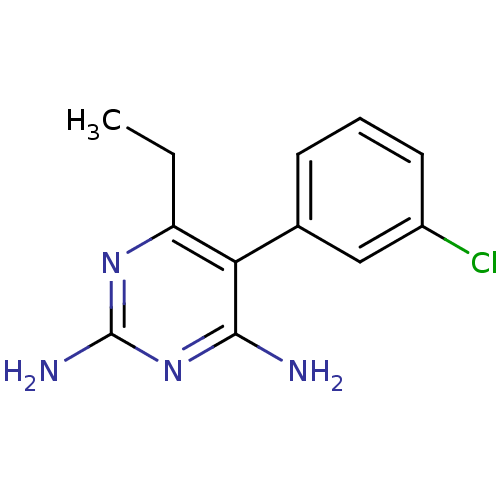 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110763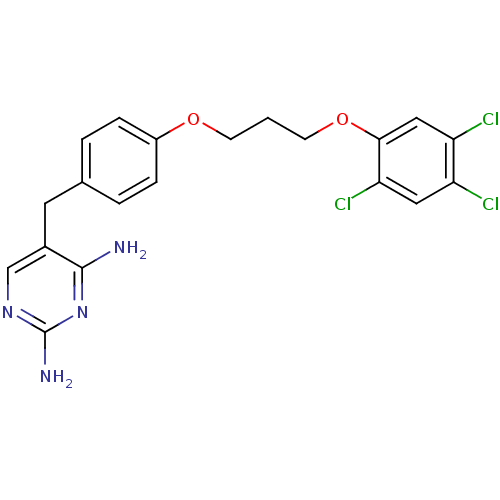 (5-{4-[3-(2,4,5-Trichloro-phenoxy)-propoxy]-benzyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18791 (5-phenyl-6-(3-phenylpropyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18780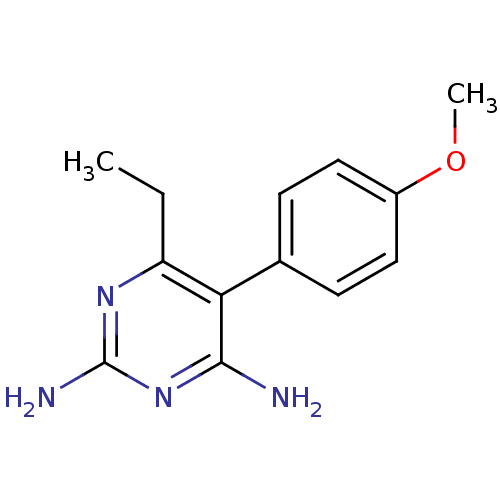 (6-ethyl-5-(4-methoxyphenyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110758 (5-(4-(benzyloxy)-3-ethoxybenzyl)pyrimidine-2,4-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138708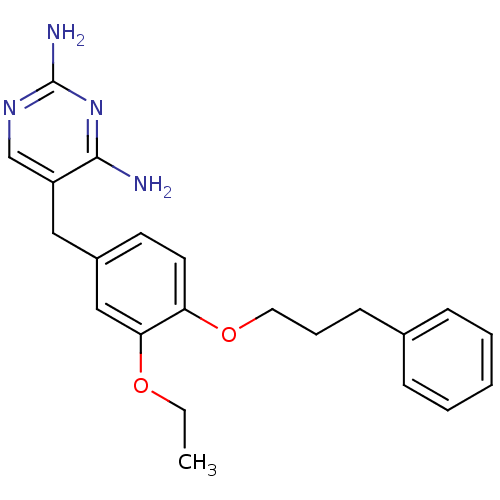 (5-[3-Ethoxy-4-(3-phenyl-propoxy)-benzyl]-pyrimidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18783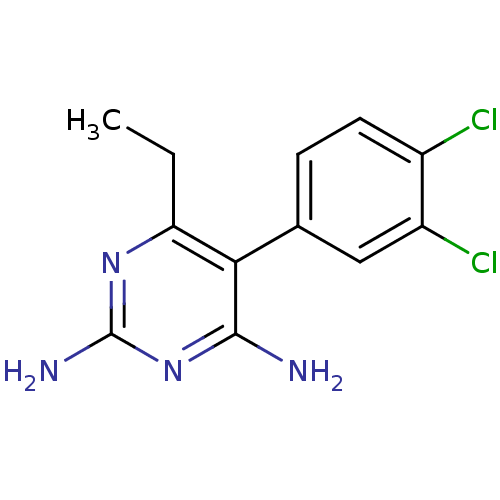 (5-(3,4-dichlorophenyl)-6-ethylpyrimidine-2,4-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110768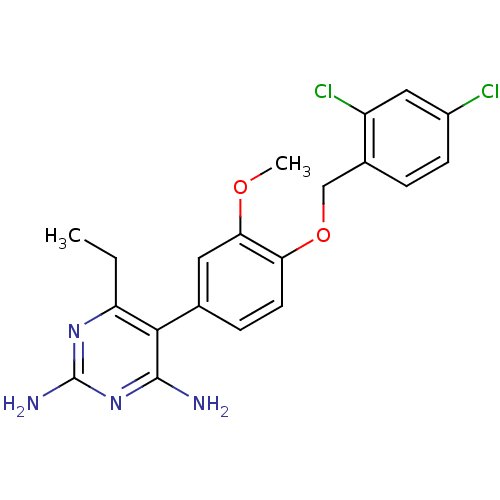 (5-[4-(2,4-Dichloro-benzyloxy)-3-methoxy-phenyl]-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110775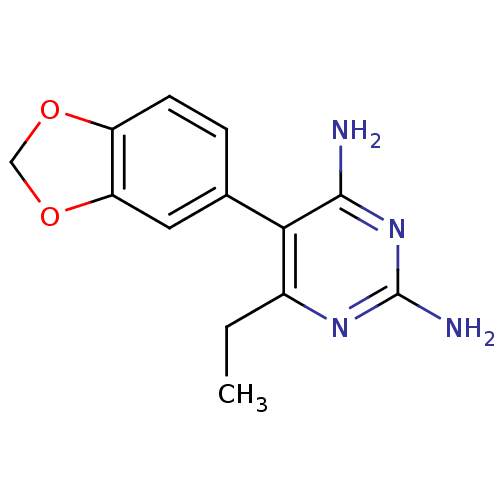 (5-Benzo[1,3]dioxol-5-yl-6-ethyl-pyrimidine-2,4-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18786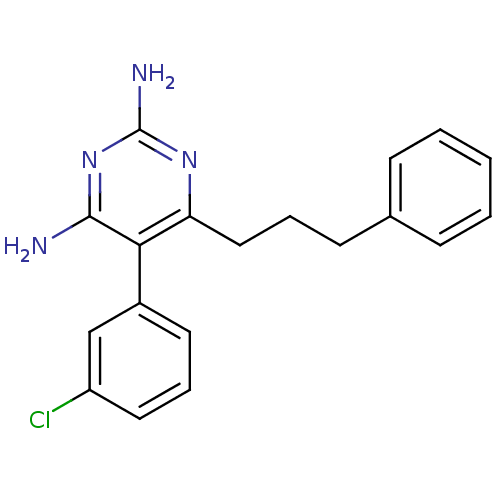 (5-(3-chlorophenyl)-6-(3-phenylpropyl)pyrimidine-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18790 (6-hexyl-5-phenylpyrimidine-2,4-diamine | CHEMBL416...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110752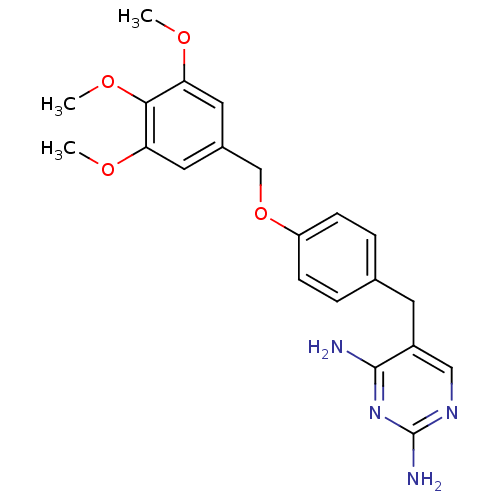 (5-(4-(3,4,5-trimethoxybenzyloxy)benzyl)pyrimidine-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110752 (5-(4-(3,4,5-trimethoxybenzyloxy)benzyl)pyrimidine-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the C59R+S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50138697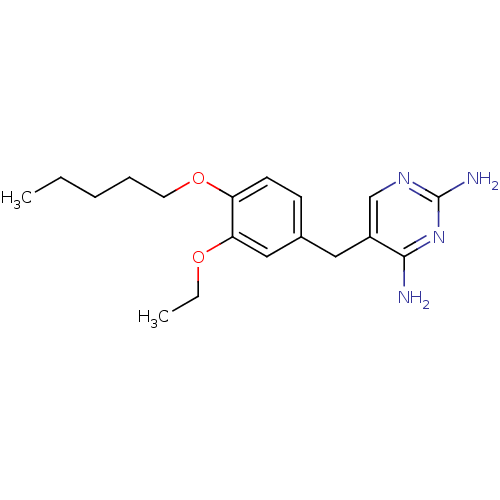 (5-(3-Ethoxy-4-pentyloxy-benzyl)-pyrimidine-2,4-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110769 (5-(3-propoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110767 (4-[2,6-Diamino-5-(3-chloro-phenyl)-pyrimidin-4-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110773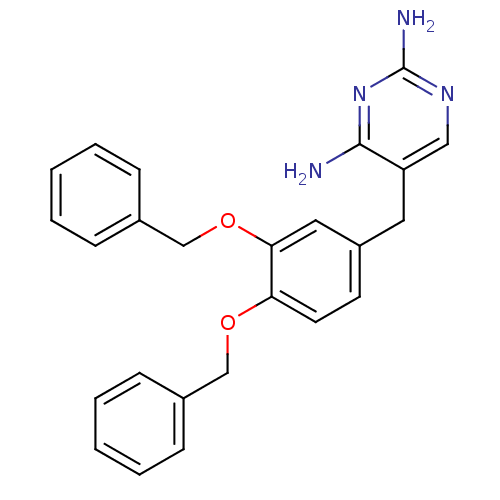 (5-(3,4-Bis-benzyloxy-benzyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110755 (5-(3-butoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110773 (5-(3,4-Bis-benzyloxy-benzyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110759 (4-(2,6-Diamino-5-phenyl-pyrimidin-4-yl)-butyric ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the S108N mutant of dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2611 total ) | Next | Last >> |