 Found 1548 hits with Last Name = 'sage' and Initial = 'c'
Found 1548 hits with Last Name = 'sage' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374104
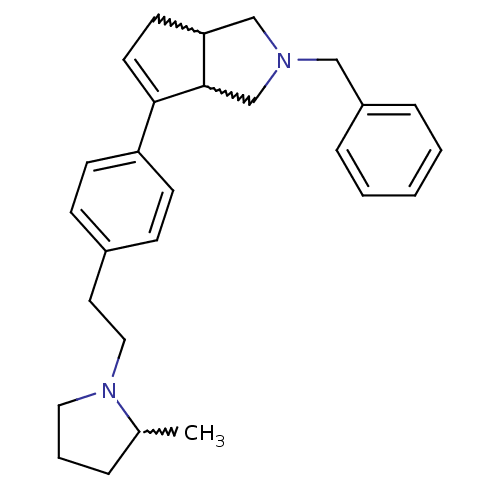
(CHEMBL255962)Show SMILES CC1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:17.18,28.30,1.0,t:16| Show InChI InChI=1S/C27H34N2/c1-21-6-5-16-29(21)17-15-22-9-11-24(12-10-22)26-14-13-25-19-28(20-27(25)26)18-23-7-3-2-4-8-23/h2-4,7-12,14,21,25,27H,5-6,13,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374100

(CHEMBL270011)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(Cc3ccccc3)C[C@@H]12)N1CCCC1 |t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2/t24-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374110

(CHEMBL401954)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(C[C@@H]12)C1CCCC1)N1CCCC1 |t:9| Show InChI InChI=1S/C24H34N2/c1-2-6-22(5-1)26-17-21-11-12-23(24(21)18-26)20-9-7-19(8-10-20)13-16-25-14-3-4-15-25/h7-10,12,21-22,24H,1-6,11,13-18H2/t21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374102
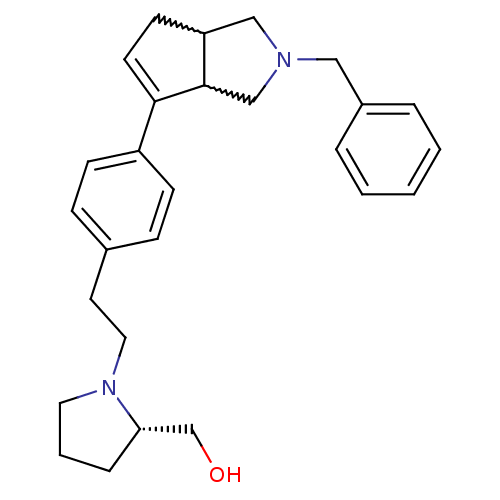
(CHEMBL402297)Show SMILES OC[C@@H]1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:18.19,29.31,t:17| Show InChI InChI=1S/C27H34N2O/c30-20-25-7-4-15-29(25)16-14-21-8-10-23(11-9-21)26-13-12-24-18-28(19-27(24)26)17-22-5-2-1-3-6-22/h1-3,5-6,8-11,13,24-25,27,30H,4,7,12,14-20H2/t24?,25-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374109

(CHEMBL255840)Show SMILES CC(C)N1C[C@@H]2CC=C([C@@H]2C1)c1ccc(CCN2CCCC2)cc1 |c:7| Show InChI InChI=1S/C22H32N2/c1-17(2)24-15-20-9-10-21(22(20)16-24)19-7-5-18(6-8-19)11-14-23-12-3-4-13-23/h5-8,10,17,20,22H,3-4,9,11-16H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374095
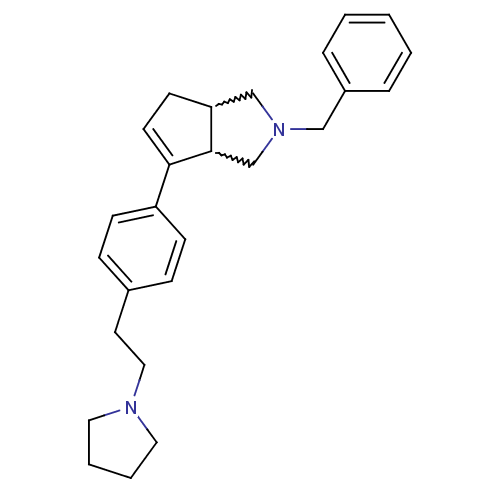
(CHEMBL255752)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCCC1 |w:11.12,22.23,t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206734
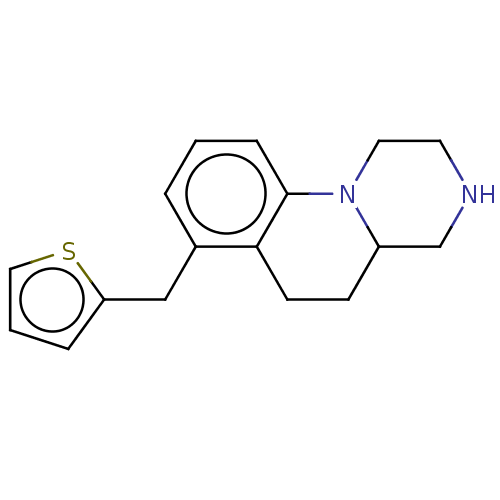
(CHEMBL3959570)Show InChI InChI=1S/C17H20N2S/c1-3-13(11-15-4-2-10-20-15)16-7-6-14-12-18-8-9-19(14)17(16)5-1/h1-5,10,14,18H,6-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206725
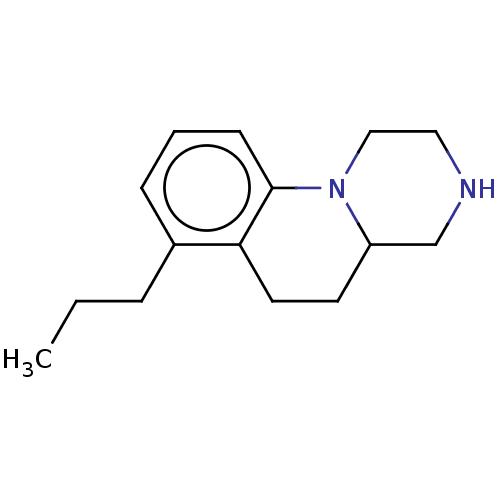
(CHEMBL3947276)Show InChI InChI=1S/C15H22N2/c1-2-4-12-5-3-6-15-14(12)8-7-13-11-16-9-10-17(13)15/h3,5-6,13,16H,2,4,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206719
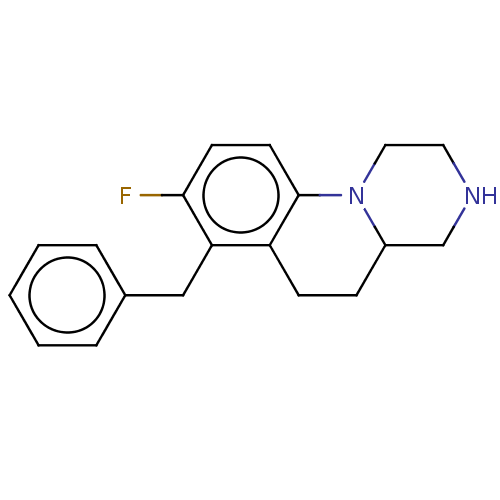
(CHEMBL3956394)Show InChI InChI=1S/C19H21FN2/c20-18-8-9-19-16(7-6-15-13-21-10-11-22(15)19)17(18)12-14-4-2-1-3-5-14/h1-5,8-9,15,21H,6-7,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374101
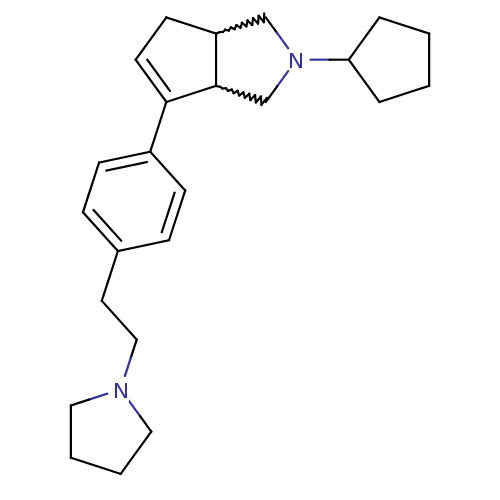
(CHEMBL410623)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(CC12)C1CCCC1)N1CCCC1 |w:11.12,15.15,t:9| Show InChI InChI=1S/C24H34N2/c1-2-6-22(5-1)26-17-21-11-12-23(24(21)18-26)20-9-7-19(8-10-20)13-16-25-14-3-4-15-25/h7-10,12,21-22,24H,1-6,11,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374098
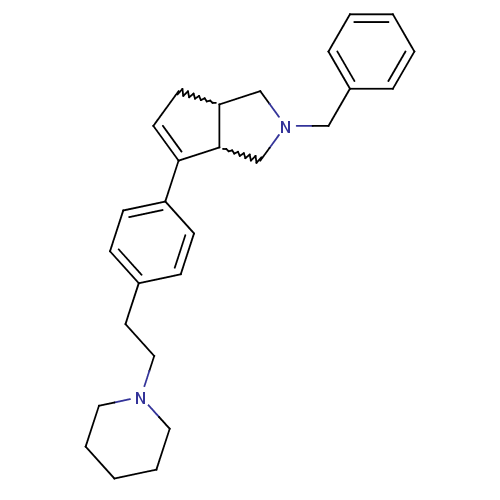
(CHEMBL256225)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCCCC1 |w:11.11,22.23,t:9| Show InChI InChI=1S/C27H34N2/c1-3-7-23(8-4-1)19-29-20-25-13-14-26(27(25)21-29)24-11-9-22(10-12-24)15-18-28-16-5-2-6-17-28/h1,3-4,7-12,14,25,27H,2,5-6,13,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374103

(CHEMBL255961)Show SMILES OC[C@H]1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:18.19,29.31,t:17| Show InChI InChI=1S/C27H34N2O/c30-20-25-7-4-15-29(25)16-14-21-8-10-23(11-9-21)26-13-12-24-18-28(19-27(24)26)17-22-5-2-1-3-6-22/h1-3,5-6,8-11,13,24-25,27,30H,4,7,12,14-20H2/t24?,25-,27?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541196

(CHEMBL4639090)Show InChI InChI=1S/C12H15BrN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206724
(CHEMBL3912721)Show InChI InChI=1S/C17H24N2/c1-3-13(4-1)11-14-5-2-6-17-16(14)8-7-15-12-18-9-10-19(15)17/h2,5-6,13,15,18H,1,3-4,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206719

(CHEMBL3956394)Show InChI InChI=1S/C19H21FN2/c20-18-8-9-19-16(7-6-15-13-21-10-11-22(15)19)17(18)12-14-4-2-1-3-5-14/h1-5,8-9,15,21H,6-7,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206714
(CHEMBL3919795)Show InChI InChI=1S/C19H22N2/c1-2-5-15(6-3-1)13-16-7-4-8-19-18(16)10-9-17-14-20-11-12-21(17)19/h1-8,17,20H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374111

(CHEMBL409899)Show SMILES C(Cc1ccc(cc1)C1=CC[C@@H]2CN(Cc3ccccc3)C[C@H]12)N1CCCC1 |t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206716
(CHEMBL3935339)Show InChI InChI=1S/C20H22N2O2/c1-2-15(10-14-4-7-19-20(11-14)24-13-23-19)17-6-5-16-12-21-8-9-22(16)18(17)3-1/h1-4,7,11,16,21H,5-6,8-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541195
(CHEMBL4634942)Show InChI InChI=1S/C12H15ClN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541197
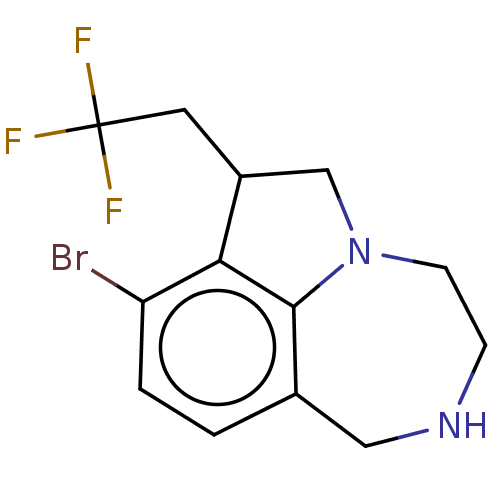
(CHEMBL4646572)Show InChI InChI=1S/C13H14BrF3N2/c14-10-2-1-8-6-18-3-4-19-7-9(5-13(15,16)17)11(10)12(8)19/h1-2,9,18H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374107
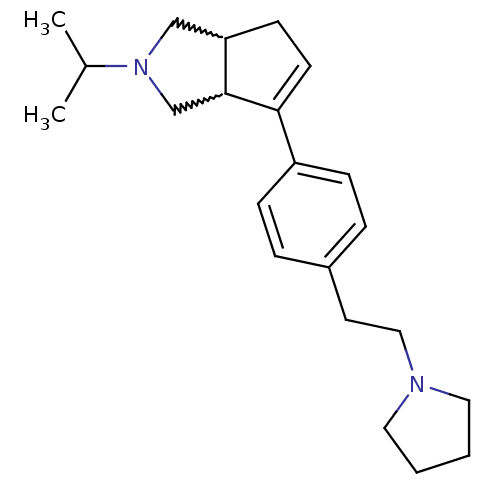
(CHEMBL270440)Show SMILES CC(C)N1CC2CC=C(C2C1)c1ccc(CCN2CCCC2)cc1 |w:5.4,9.10,c:7| Show InChI InChI=1S/C22H32N2/c1-17(2)24-15-20-9-10-21(22(20)16-24)19-7-5-18(6-8-19)11-14-23-12-3-4-13-23/h5-8,10,17,20,22H,3-4,9,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541204

(CHEMBL4636977)Show InChI InChI=1S/C13H17BrN2/c1-13(2)8-16-6-5-15-7-9-3-4-10(14)11(13)12(9)16/h3-4,15H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541205

(CHEMBL4635105)Show InChI InChI=1S/C13H17ClN2/c1-13(2)8-16-6-5-15-7-9-3-4-10(14)11(13)12(9)16/h3-4,15H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206714

(CHEMBL3919795)Show InChI InChI=1S/C19H22N2/c1-2-5-15(6-3-1)13-16-7-4-8-19-18(16)10-9-17-14-20-11-12-21(17)19/h1-8,17,20H,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206716

(CHEMBL3935339)Show InChI InChI=1S/C20H22N2O2/c1-2-15(10-14-4-7-19-20(11-14)24-13-23-19)17-6-5-16-12-21-8-9-22(16)18(17)3-1/h1-4,7,11,16,21H,5-6,8-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374097

(CHEMBL255166)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCOCC1 |w:11.11,22.23,t:9| Show InChI InChI=1S/C26H32N2O/c1-2-4-22(5-3-1)18-28-19-24-10-11-25(26(24)20-28)23-8-6-21(7-9-23)12-13-27-14-16-29-17-15-27/h1-9,11,24,26H,10,12-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374105
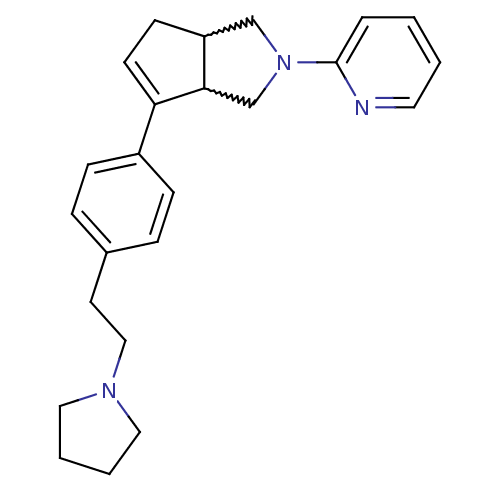
(CHEMBL429418)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(CC12)c1ccccn1)N1CCCC1 |w:11.12,15.15,t:9| Show InChI InChI=1S/C24H29N3/c1-2-13-25-24(5-1)27-17-21-10-11-22(23(21)18-27)20-8-6-19(7-9-20)12-16-26-14-3-4-15-26/h1-2,5-9,11,13,21,23H,3-4,10,12,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374108
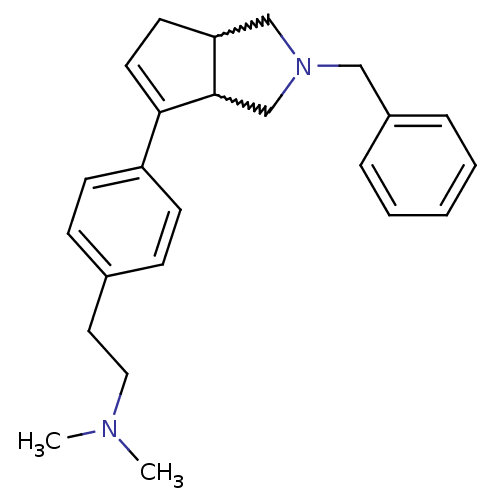
(CHEMBL273202)Show SMILES CN(C)CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:14.15,25.26,t:12| Show InChI InChI=1S/C24H30N2/c1-25(2)15-14-19-8-10-21(11-9-19)23-13-12-22-17-26(18-24(22)23)16-20-6-4-3-5-7-20/h3-11,13,22,24H,12,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541194
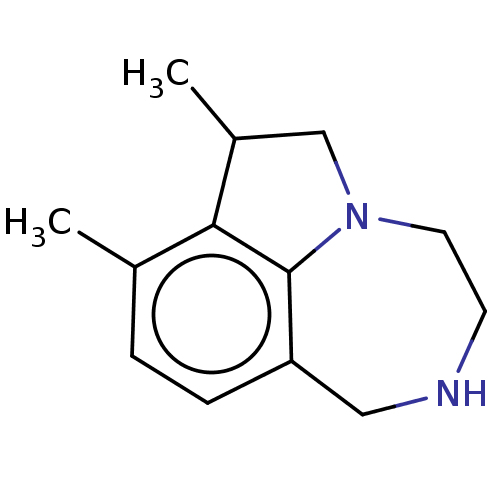
(CHEMBL4646618)Show InChI InChI=1S/C13H18N2/c1-9-3-4-11-7-14-5-6-15-8-10(2)12(9)13(11)15/h3-4,10,14H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374099
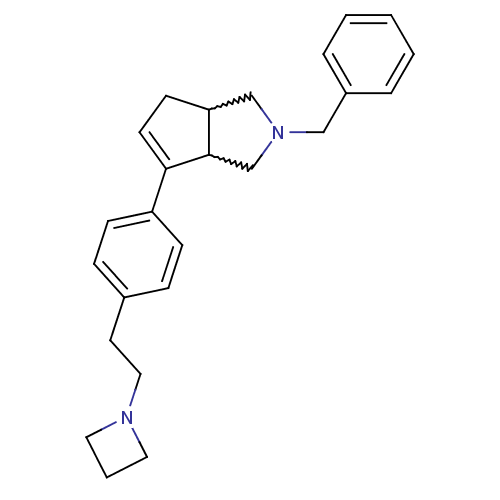
(CHEMBL256648)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCC1 |w:11.12,22.23,t:9| Show InChI InChI=1S/C25H30N2/c1-2-5-21(6-3-1)17-27-18-23-11-12-24(25(23)19-27)22-9-7-20(8-10-22)13-16-26-14-4-15-26/h1-3,5-10,12,23,25H,4,11,13-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541199
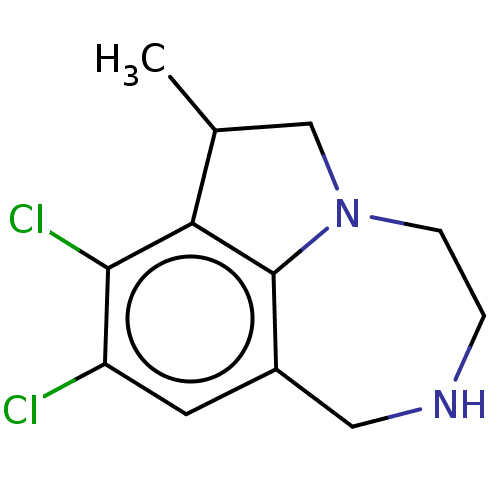
(CHEMBL4632473)Show InChI InChI=1S/C12H14Cl2N2/c1-7-6-16-3-2-15-5-8-4-9(13)11(14)10(7)12(8)16/h4,7,15H,2-3,5-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374094

(CHEMBL402296)Show SMILES FC1(F)CCN(CCc2ccc(cc2)C2=CCC3CN(Cc4ccccc4)CC23)C1 |w:17.17,28.29,t:15| Show InChI InChI=1S/C26H30F2N2/c27-26(28)13-15-29(19-26)14-12-20-6-8-22(9-7-20)24-11-10-23-17-30(18-25(23)24)16-21-4-2-1-3-5-21/h1-9,11,23,25H,10,12-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541208

(CHEMBL4634785)Show SMILES [H][C@@]12CCC[C@]1([H])C1=CC=CC3CNCCN(C2)C13 |r,c:10,t:8| Show InChI InChI=1S/C15H22N2/c1-3-11-9-16-7-8-17-10-12-4-2-5-13(12)14(6-1)15(11)17/h1,3,6,11-13,15-16H,2,4-5,7-10H2/t11?,12-,13-,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from recombinant human 5HT2C receptor expressed in CHO cells measured after 120 ... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541207
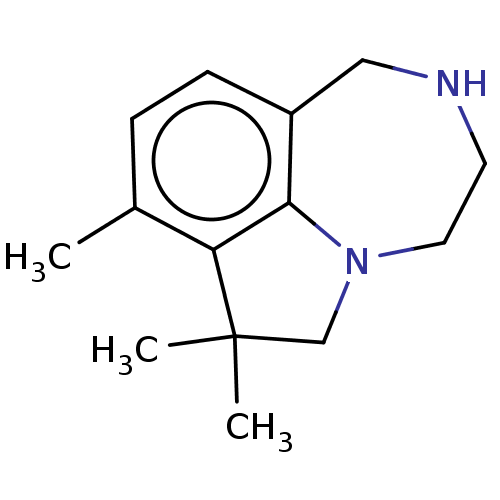
(CHEMBL4638879)Show InChI InChI=1S/C14H20N2/c1-10-4-5-11-8-15-6-7-16-9-14(2,3)12(10)13(11)16/h4-5,15H,6-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541177
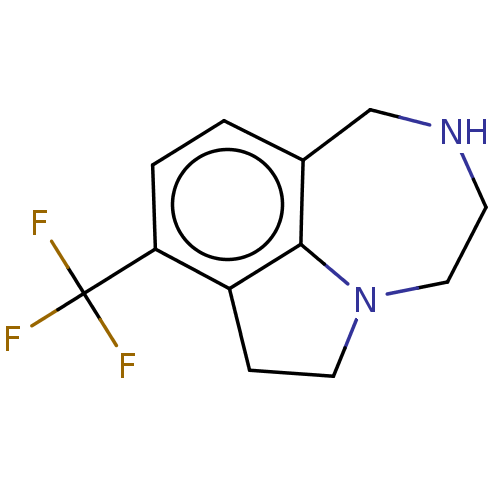
(CHEMBL4647417)Show InChI InChI=1S/C12H13F3N2/c13-12(14,15)10-2-1-8-7-16-4-6-17-5-3-9(10)11(8)17/h1-2,16H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206725

(CHEMBL3947276)Show InChI InChI=1S/C15H22N2/c1-2-4-12-5-3-6-15-14(12)8-7-13-11-16-9-10-17(13)15/h3,5-6,13,16H,2,4,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor (unknown origin) expressed in HEK293 cells assessed as [3H]inositol phosphate accumulation after 2 hrs by scintil... |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50206718
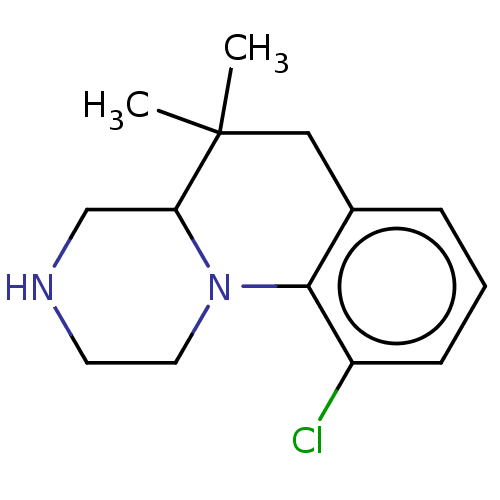
(CHEMBL3895972)Show InChI InChI=1S/C14H19ClN2/c1-14(2)8-10-4-3-5-11(15)13(10)17-7-6-16-9-12(14)17/h3-5,12,16H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2B receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374112

(CHEMBL258302)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(CC12)c1cncnc1)N1CCCC1 |w:11.12,15.15,t:9| Show InChI InChI=1S/C23H28N4/c1-2-11-26(10-1)12-9-18-3-5-19(6-4-18)22-8-7-20-15-27(16-23(20)22)21-13-24-17-25-14-21/h3-6,8,13-14,17,20,23H,1-2,7,9-12,15-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235370
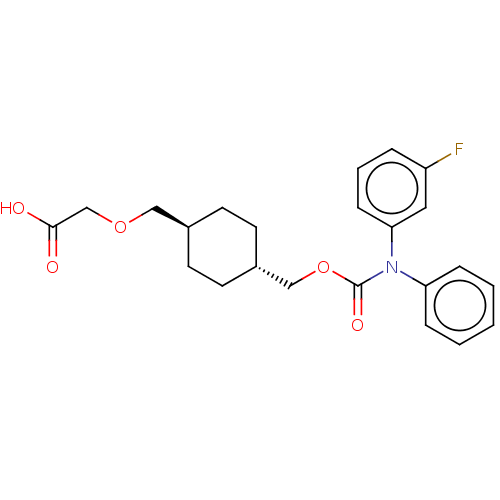
(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541176
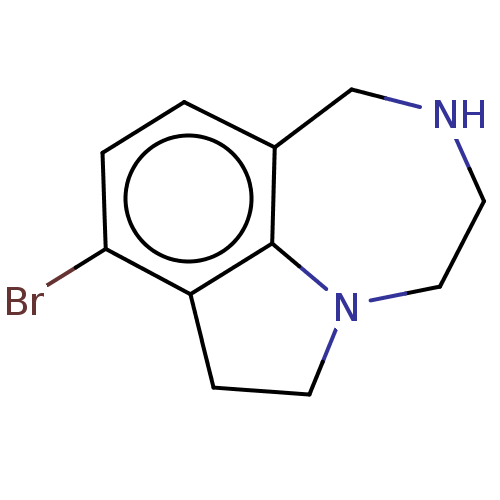
(CHEMBL4635854)Show InChI InChI=1S/C11H13BrN2/c12-10-2-1-8-7-13-4-6-14-5-3-9(10)11(8)14/h1-2,13H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206724

(CHEMBL3912721)Show InChI InChI=1S/C17H24N2/c1-3-13(4-1)11-14-5-2-6-17-16(14)8-7-15-12-18-9-10-19(15)17/h2,5-6,13,15,18H,1,3-4,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001915
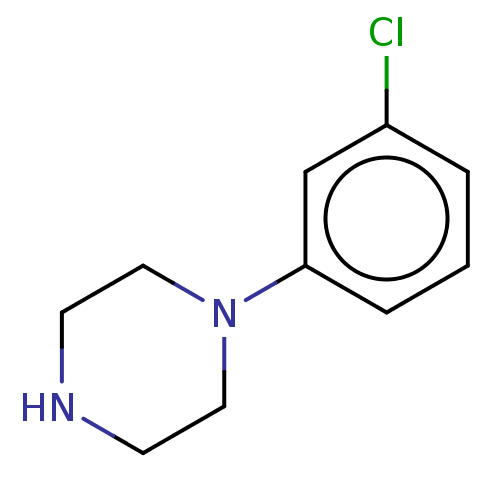
(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541200
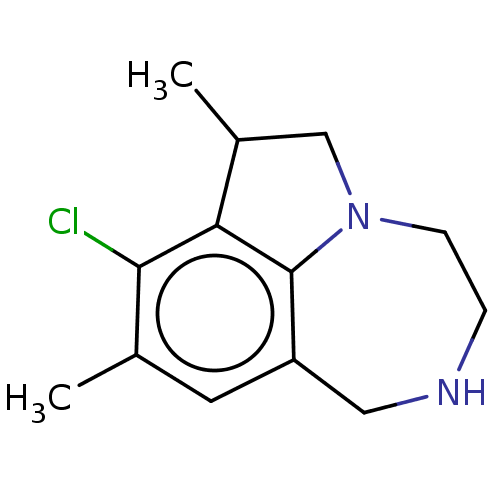
(CHEMBL4644872)Show InChI InChI=1S/C13H17ClN2/c1-8-5-10-6-15-3-4-16-7-9(2)11(12(8)14)13(10)16/h5,9,15H,3-4,6-7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001915

(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2B receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541168
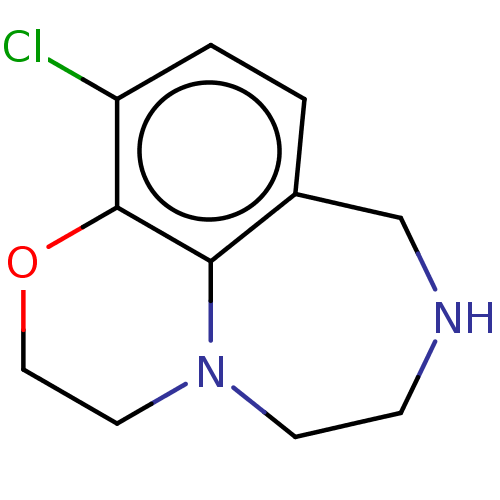
(CHEMBL4648457)Show InChI InChI=1S/C11H13ClN2O/c12-9-2-1-8-7-13-3-4-14-5-6-15-11(9)10(8)14/h1-2,13H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data