

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016904 (CHEMBL3273012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016905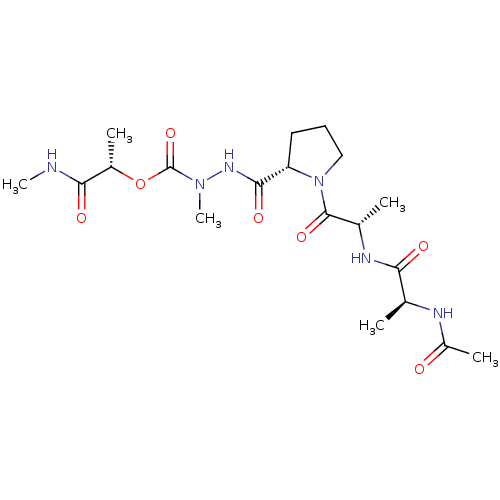 (CHEMBL3273010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016903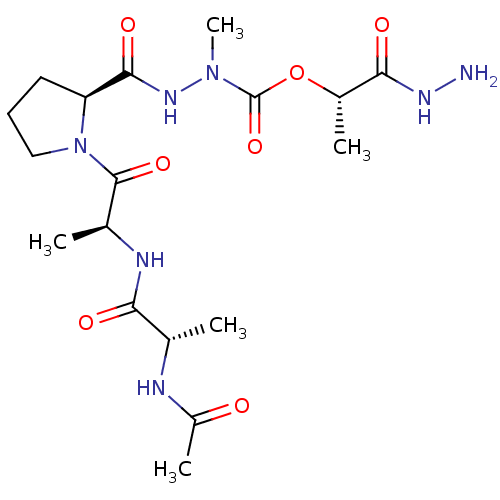 (CHEMBL3273013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016930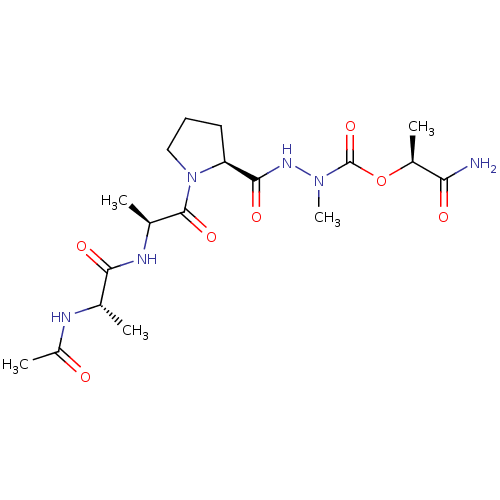 (CHEMBL3273008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016906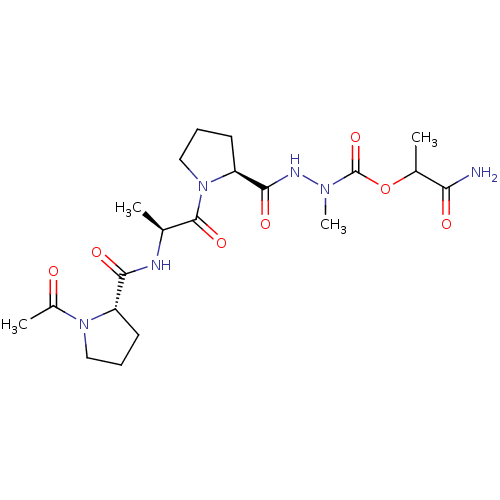 (CHEMBL3273009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50016929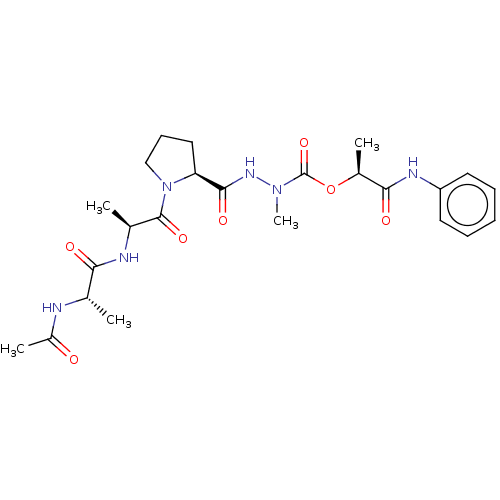 (CHEMBL3273011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of human granulocyte elastase using Ac-Ala-Ala-Pro-Ala-p-nitroanilide as substrate by Dixon plot analysis | J Med Chem 20: 1464-8 (1977) BindingDB Entry DOI: 10.7270/Q2WQ05BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136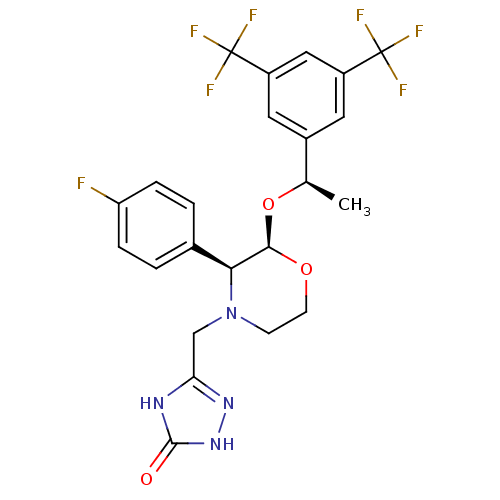 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compounds were evaluated for inhibitory activity against human Tachykinin receptor 1 | J Med Chem 43: 1234-41 (2000) BindingDB Entry DOI: 10.7270/Q2G73CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096519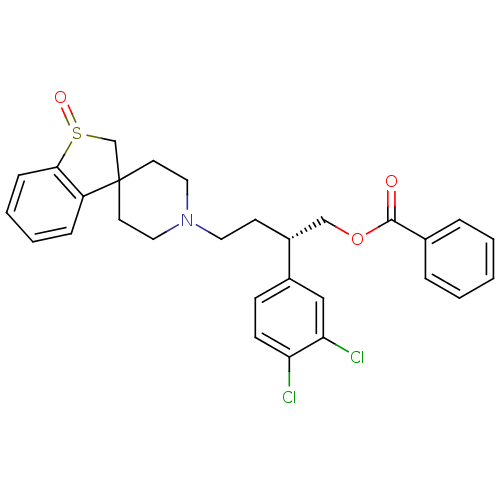 (Benzoic acid (S)-2-(3,4-dichloro-phenyl)-4-(spiro[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand at a concentration of 1000 ... | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096520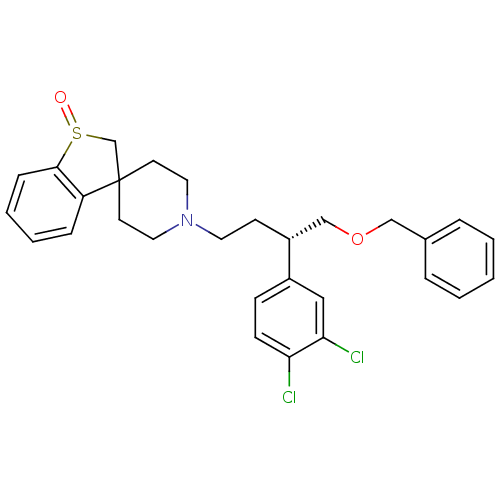 (Benzyl (S)-2-(3,4-dichloro-phenyl)-4-(spiro[1-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand at a concentration of 1000 ... | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281196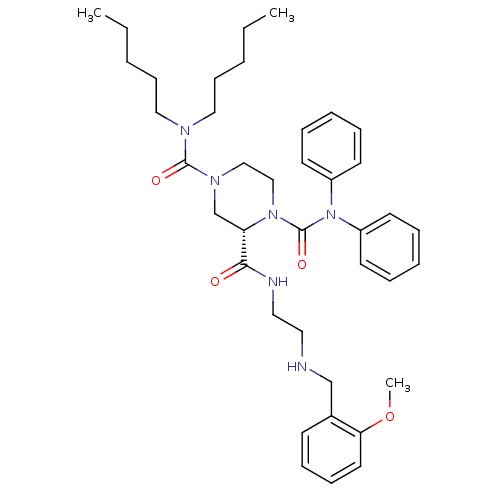 ((S)-Piperazine-1,2,4-tricarboxylic acid 4-dipentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281189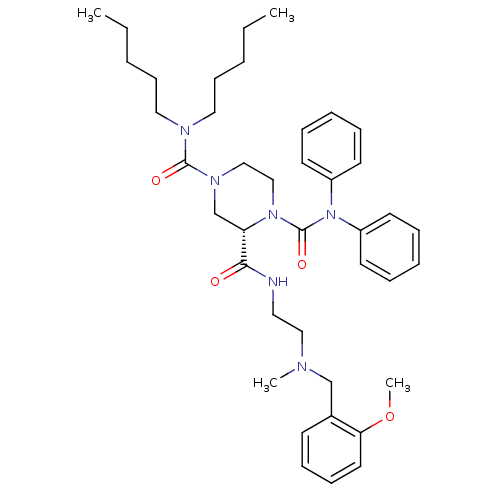 ((S)-Piperazine-1,2,4-tricarboxylic acid 4-dipentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281194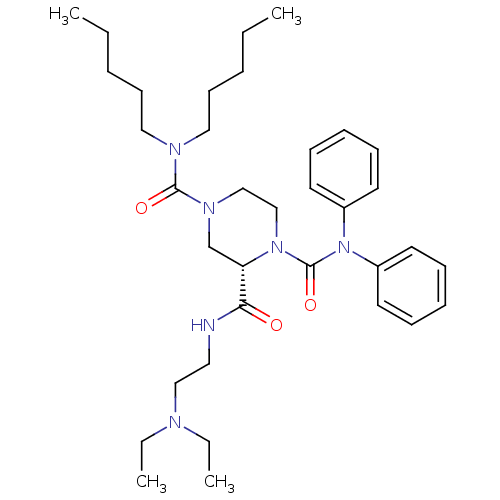 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(2-diet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281198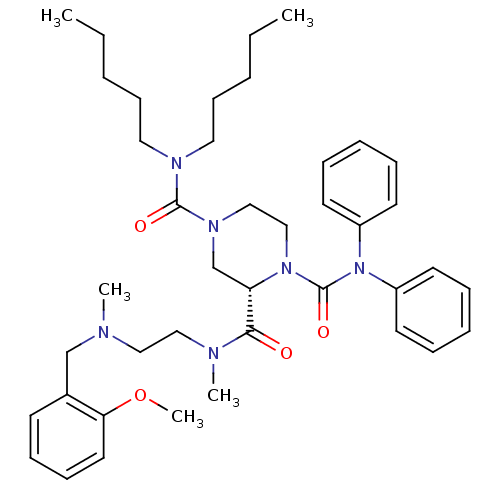 ((S)-Piperazine-1,2,4-tricarboxylic acid 4-dipentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096515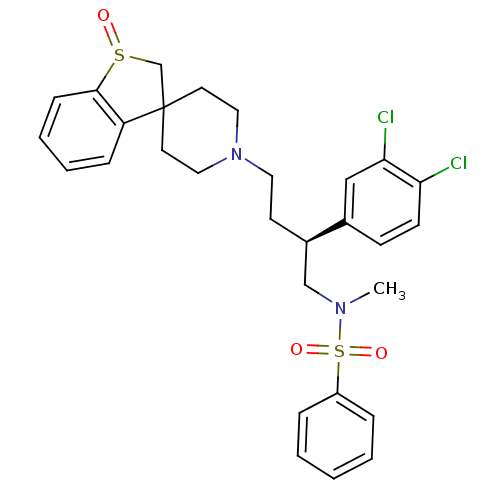 ((2S)-2-(3,4-dichlorophenyl)-1-[(N-methyl-N-phenyls...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096506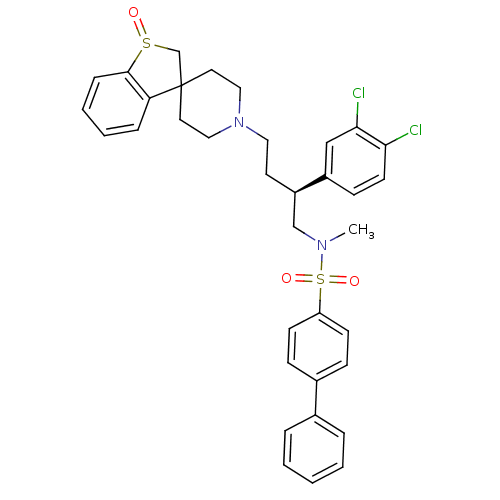 (1'-[3-(3,4-dichlorophenyl)-4-methyl(4-phenylphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096535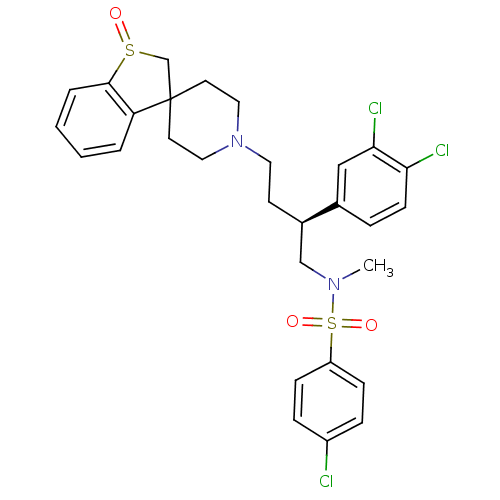 (1'-[4-[4-chlorophenyl(methyl)sulfonamido]-3-(3,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015885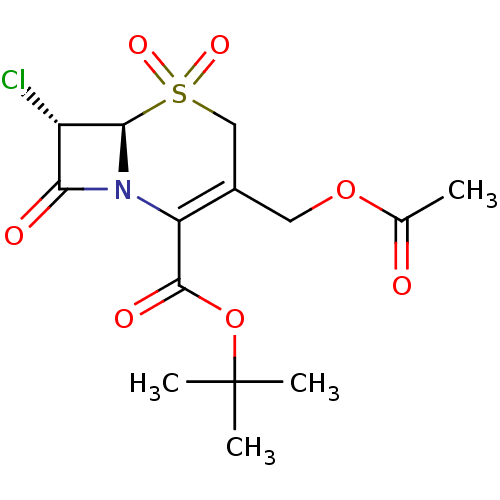 (3-Acetoxymethyl-7-chloro-5,5,8-trioxo-5lambda*6*-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration which is required to cause 50% inhibition of human leukocyte elastase (HLE) enzyme | J Med Chem 33: 2513-21 (1990) BindingDB Entry DOI: 10.7270/Q2Q81C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096539 (1'-[3-(3,4-dichlorophenyl)-4-[4-methoxyphenyl(meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281187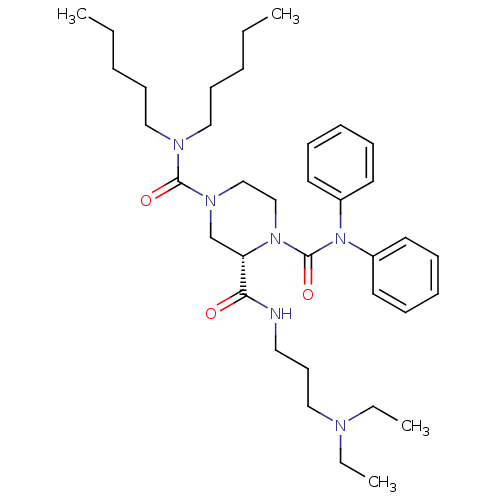 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(3-diet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281200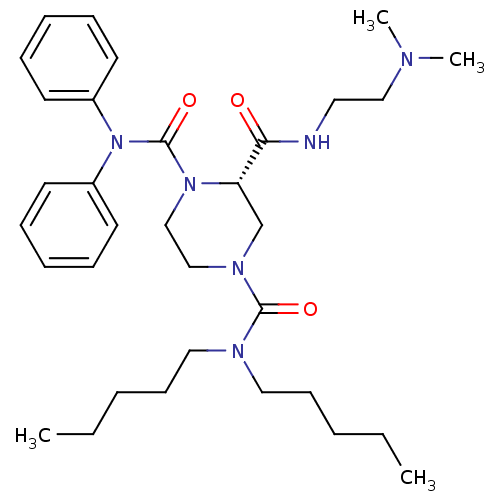 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(2-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281201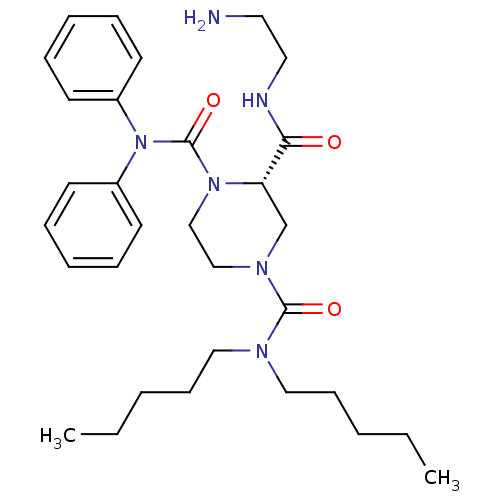 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(2-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096509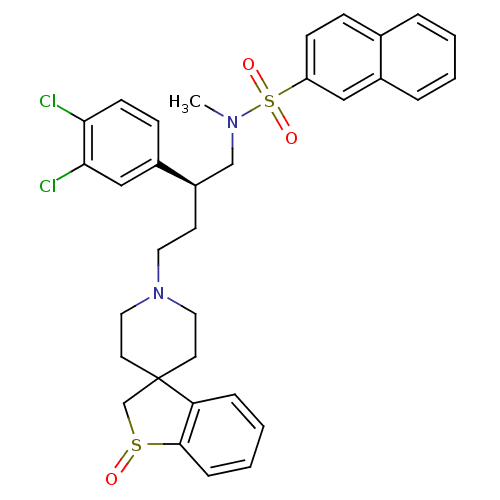 (1'-[3-(3,4-dichlorophenyl)-4-methyl(2-naphthyl)sul...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015871 (3-Acetoxymethyl-7-fluoro-5,5,8-trioxo-5lambda*6*-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration which is required to cause 50% inhibition of human leukocyte elastase (HLE) enzyme | J Med Chem 33: 2513-21 (1990) BindingDB Entry DOI: 10.7270/Q2Q81C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096529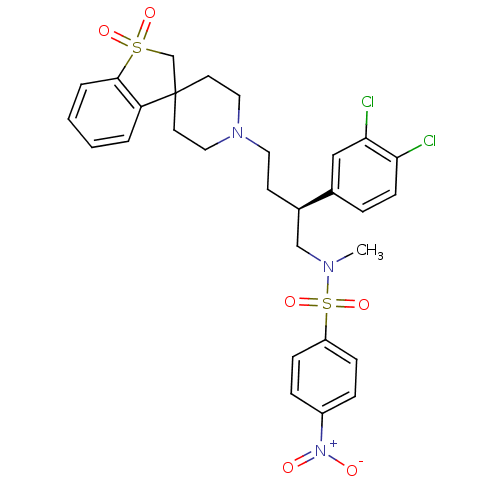 (1'-[3-(3,4-dichlorophenyl)-4-methyl(4-nitrophenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096536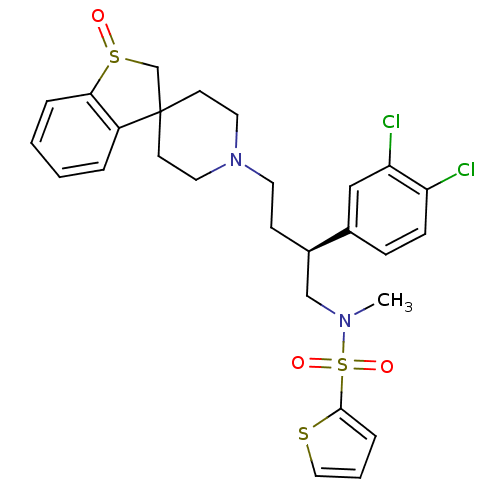 (1'-[3-(3,4-dichlorophenyl)-4-methyl(2-thienyl)sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096534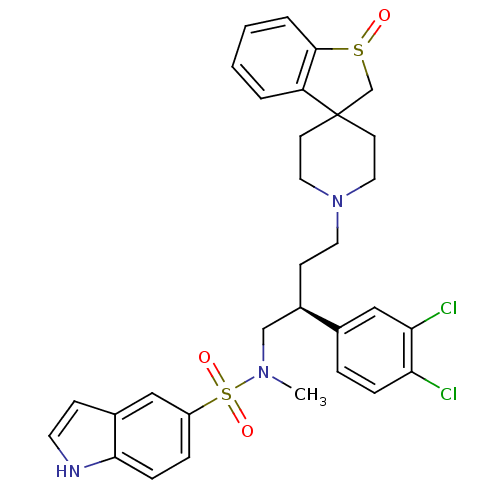 (1'-[3-(3,4-dichlorophenyl)-4-[1H-5-indolyl(methyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096537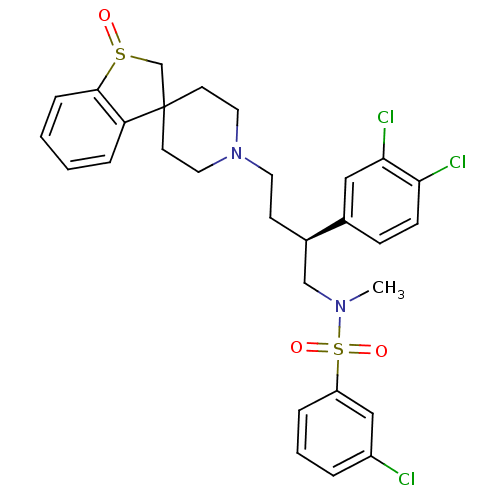 (1'-[4-[3-chlorophenyl(methyl)sulfonamido]-3-(3,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096530 (1'-[4-[2-chlorophenyl(methyl)sulfonamido]-3-(3,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096516 (1'-[3-(3,4-dichlorophenyl)-4-methyl(8-quinolyl)sul...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Ovis aries (Sheep)) | BDBM50226339 (CHEMBL33378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes | J Med Chem 29: 1436-41 (1986) BindingDB Entry DOI: 10.7270/Q2X06974 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096512 (1'-[3-(3,4-dichlorophenyl)-4-methyl(phenyl)sulfona...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Ovis aries (Sheep)) | BDBM50226348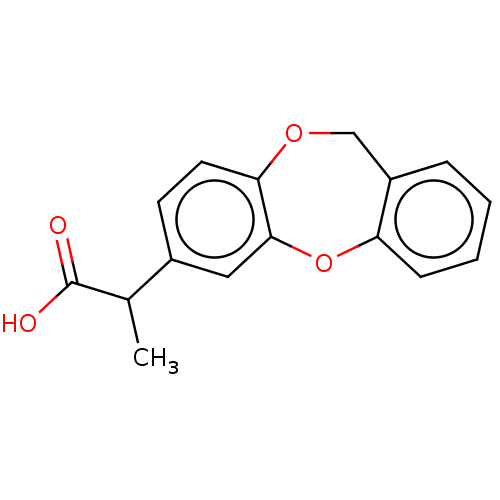 (CHEMBL284705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase of ram seminal vesicle microsomes | J Med Chem 29: 1436-41 (1986) BindingDB Entry DOI: 10.7270/Q2X06974 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096508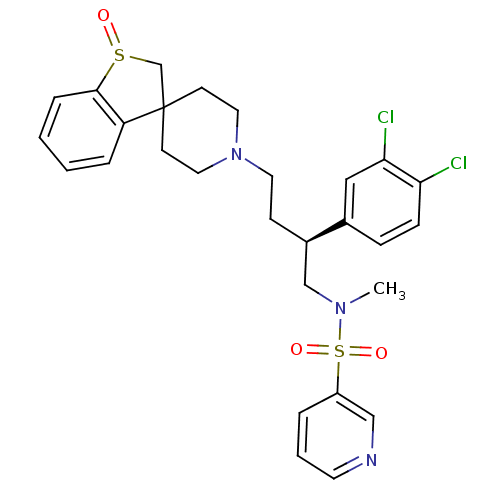 (1'-[3-(3,4-dichlorophenyl)-4-methyl(3-pyridyl)sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096514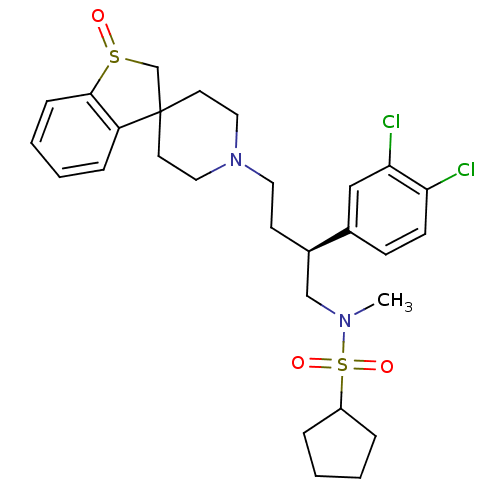 (1N-[2-(3,4-dichlorophenyl)-4-spiro[2,3-dihydrobenz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096528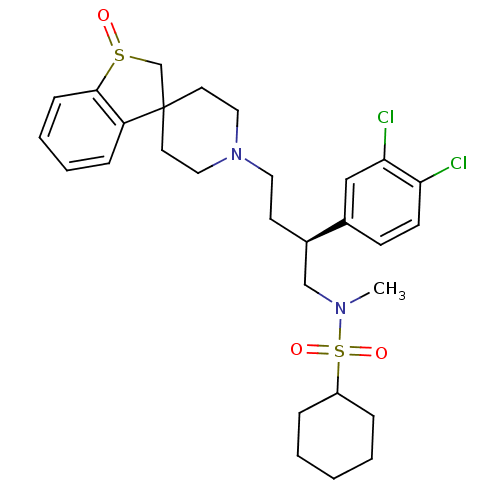 (1'-[4-cyclohexyl(methyl)sulfonamido-3-(3,4-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096511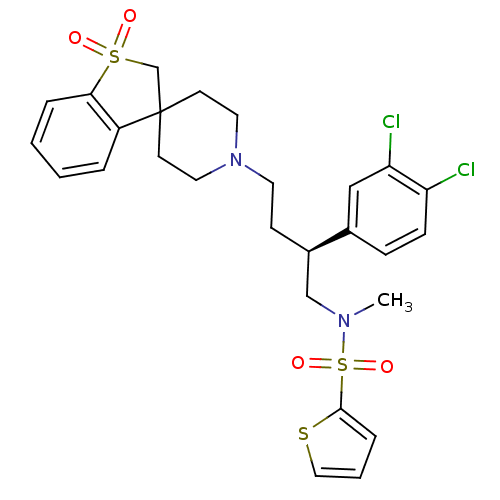 (1'-[3-(3,4-dichlorophenyl)-4-methyl(2-thienyl)sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096532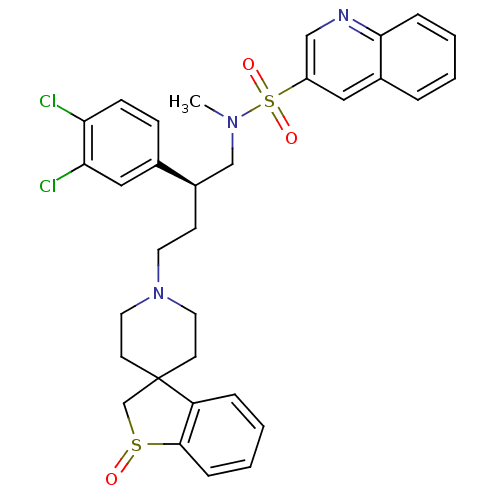 (1'-[3-(3,4-dichlorophenyl)-4-methyl(3-quinolyl)sul...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096523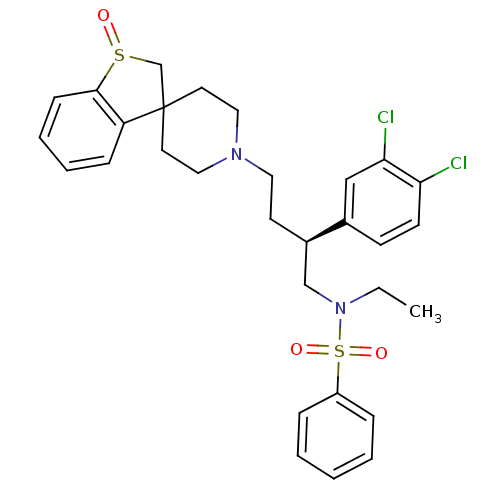 (1'-[3-(3,4-dichlorophenyl)-4-ethyl(phenyl)sulfonam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50280912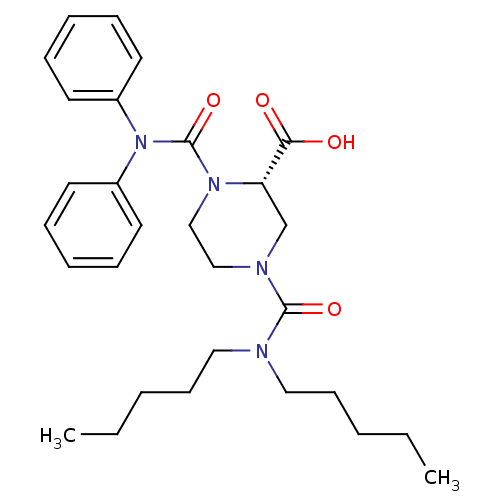 ((S)-4-Dipentylcarbamoyl-1-diphenylcarbamoyl-pipera...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281192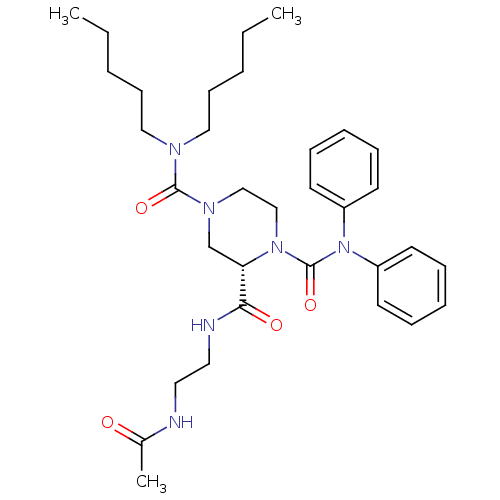 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(2-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096538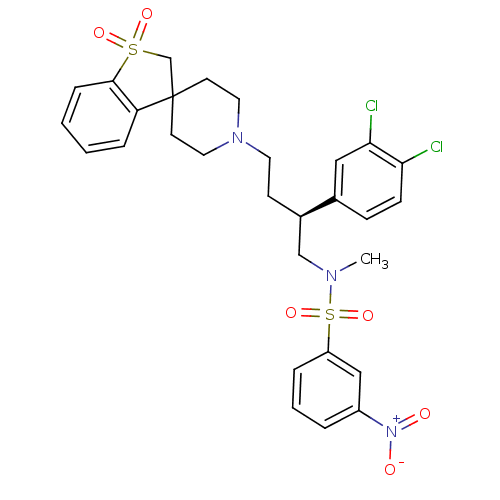 (1'-[3-(3,4-dichlorophenyl)-4-methyl(3-nitrophenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096527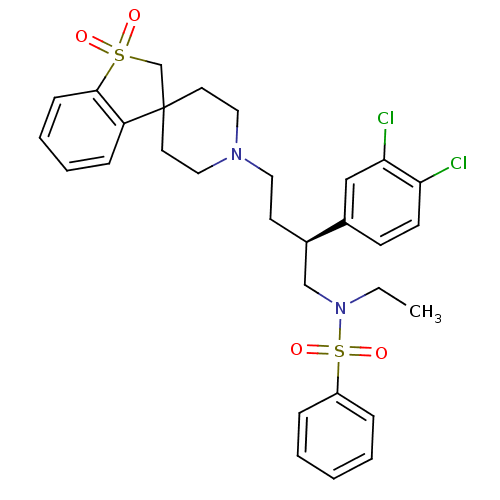 (1'-[3-(3,4-dichlorophenyl)-4-ethyl(phenyl)sulfonam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281193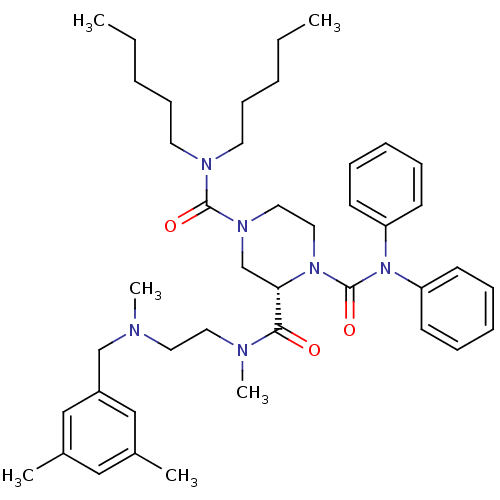 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-({2-[(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281191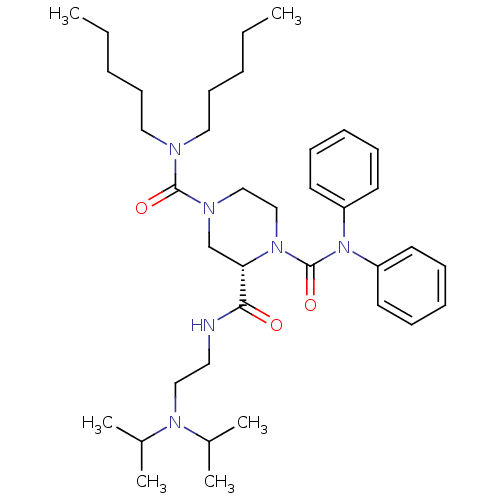 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-[(2-diis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50096505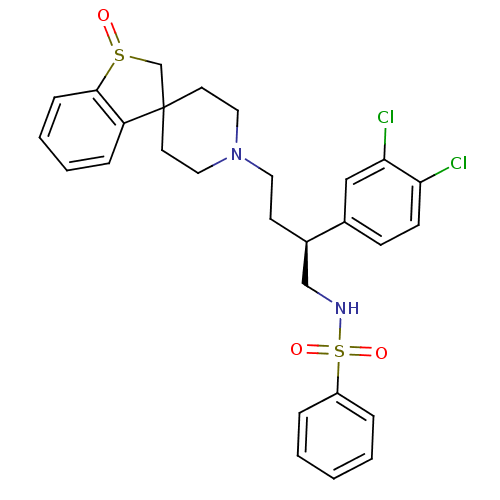 (1'-[3-(3,4-dichlorophenyl)-4-phenylsulfonamido-(3S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human CCR5 receptor in Chinese hamster ovary (CHO) cells using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 11: 259-64 (2001) BindingDB Entry DOI: 10.7270/Q29Z945C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281188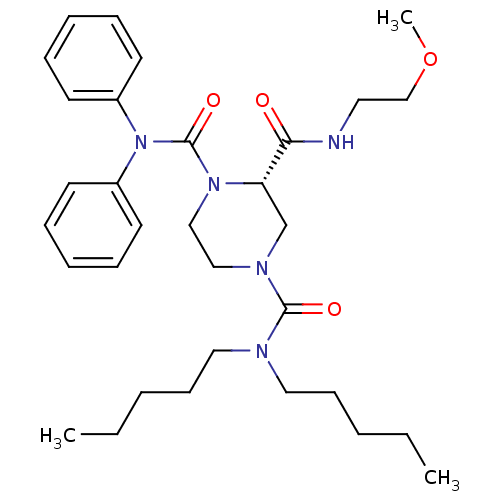 ((S)-Piperazine-1,2,4-tricarboxylic acid 4-dipentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50280905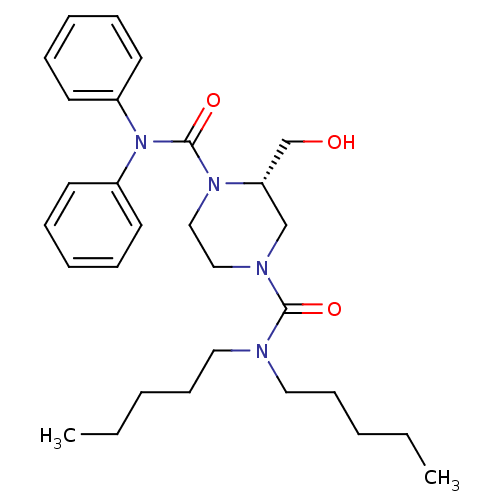 ((S)-2-Hydroxymethyl-piperazine-1,4-dicarboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50281199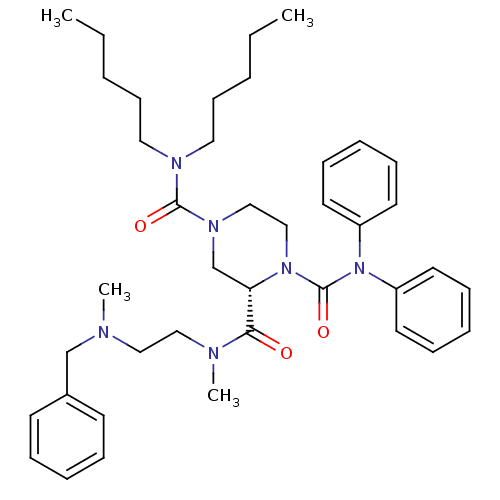 ((S)-Piperazine-1,2,4-tricarboxylic acid 2-{[2-(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Substance P binding to human NK1 receptors in CHO cells | Bioorg Med Chem Lett 3: 2707-2712 (1993) Article DOI: 10.1016/S0960-894X(01)80747-2 BindingDB Entry DOI: 10.7270/Q23F4PKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015856 (3-Acetoxymethyl-7-formyloxy-5,5,8-trioxo-5lambda*6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration which is required to cause 50% inhibition of human leukocyte elastase (HLE) enzyme | J Med Chem 33: 2513-21 (1990) BindingDB Entry DOI: 10.7270/Q2Q81C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |