 Found 36 hits with Last Name = 'castagnolo' and Initial = 'd'
Found 36 hits with Last Name = 'castagnolo' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acyl-CoA (8-3)-desaturase
(Homo sapiens (Human)) | BDBM50615159

(CHEMBL5278664)Show SMILES C[C@@H]1[C@H](OC(=O)N1c1cc(cc(c1)C#N)C#N)c1ccc(F)cc1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Decaprenylphosphoryl-beta-D-ribose oxidase
(Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50517949
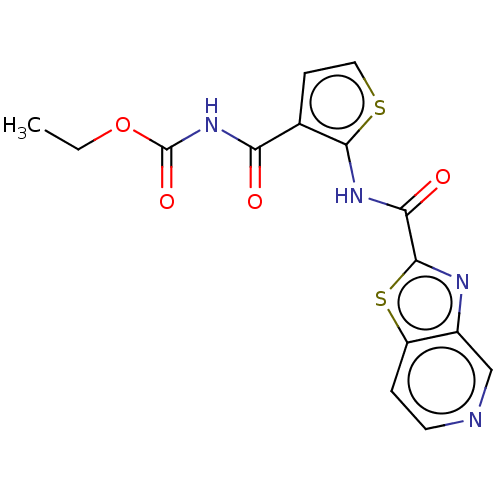
(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50589215
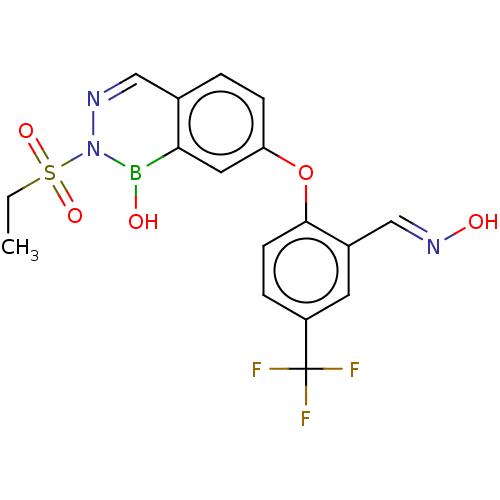
(CHEMBL5190515)Show SMILES CCS(=O)(=O)N1N=Cc2ccc(Oc3ccc(cc3\C=N\O)C(F)(F)F)cc2B1O |c:6| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517949

(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyketide synthase Pks13
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520948
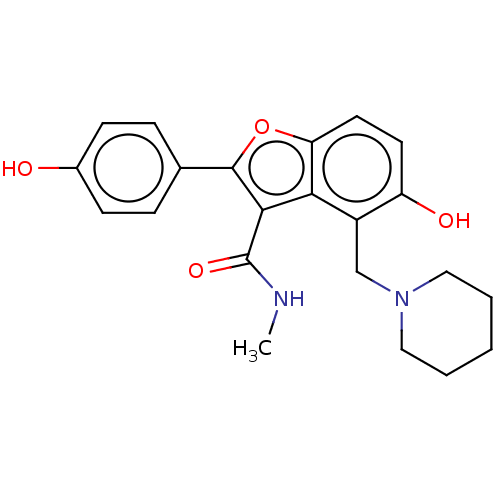
(CHEMBL4443524)Show SMILES CNC(=O)c1c(oc2ccc(O)c(CN3CCCCC3)c12)-c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O4/c1-23-22(27)20-19-16(13-24-11-3-2-4-12-24)17(26)9-10-18(19)28-21(20)14-5-7-15(25)8-6-14/h5-10,25-26H,2-4,11-13H2,1H3,(H,23,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50598090
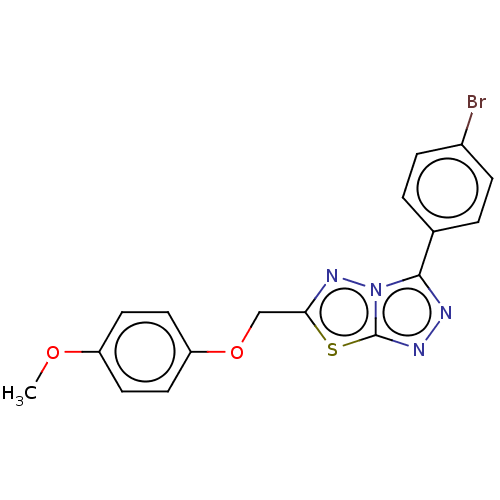
(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50598090

(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50598090

(CHEMBL5207531) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50615160

(CHEMBL5289568)Show SMILES COc1ccc(cc1)C1CC2(CN(CC(O)=O)C(=O)O2)c2cc(Br)ccc2O1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16312

((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...)Show InChI InChI=1S/C11H9FN2O3/c12-6-1-2-8-7(5-6)11(3-4-17-8)9(15)13-10(16)14-11/h1-2,5H,3-4H2,(H2,13,14,15,16)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom... |
ACS Med Chem Lett 1: 416-421 (2010)
Article DOI: 10.1021/ml100118k
BindingDB Entry DOI: 10.7270/Q28K7B2N |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598093
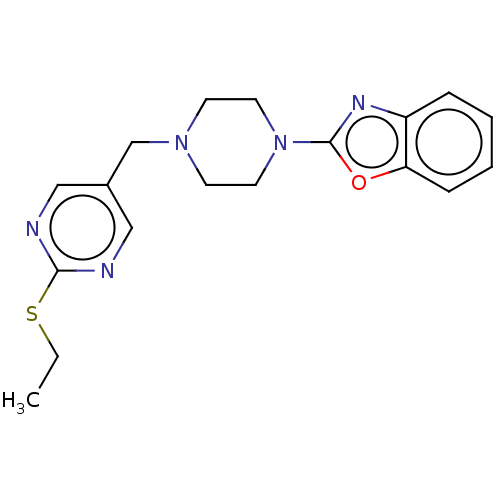
(CHEMBL5199365) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598086
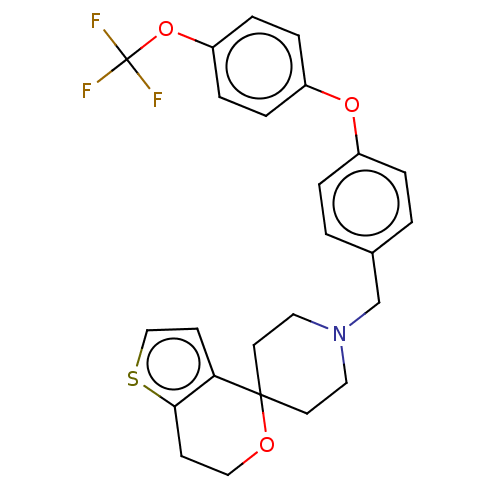
(CHEMBL5181337)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(CN3CCC4(CC3)OCCc3sccc43)cc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50615161

(CHEMBL5279118)Show SMILES COc1cc(COc2ccccc2C2(C)CN(CC(O)=O)C(=O)O2)cc(OC)c1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
O43924/P16499/P18545/P35913/P51160/Q13956
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50378908
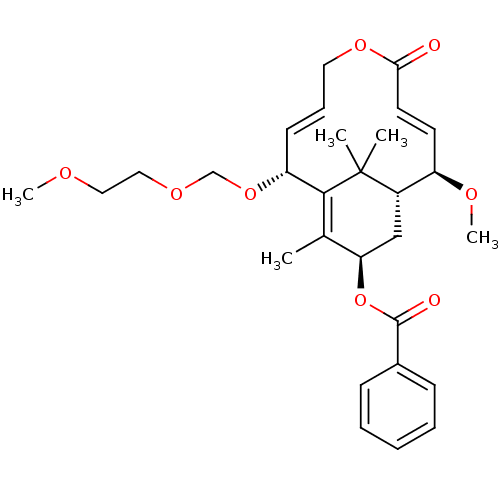
(CHEMBL1688197)Show SMILES COCCOCO[C@@H]1\C=C/COC(=O)\C=C\[C@@H](OC)[C@@H]2C[C@@H](OC(=O)c3ccccc3)C(C)=C1C2(C)C |r,c:8,t:14,33| Show InChI InChI=1S/C29H38O8/c1-20-25(37-28(31)21-10-7-6-8-11-21)18-22-23(33-5)13-14-26(30)35-15-9-12-24(27(20)29(22,2)3)36-19-34-17-16-32-4/h6-14,22-25H,15-19H2,1-5H3/b12-9-,14-13+/t22-,23+,24+,25+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom... |
ACS Med Chem Lett 1: 416-421 (2010)
Article DOI: 10.1021/ml100118k
BindingDB Entry DOI: 10.7270/Q28K7B2N |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50366826
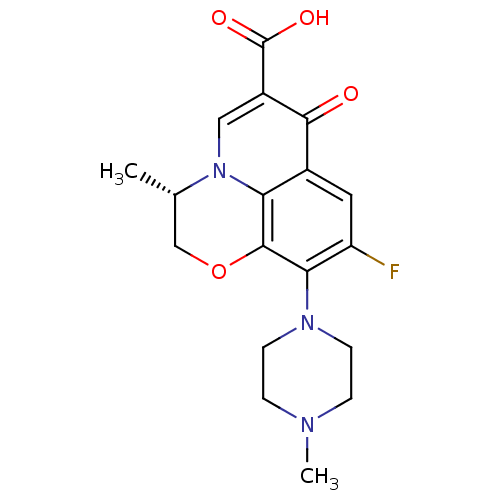
(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus topoisomerase-4 expressed in Escherichia coli assessed as relaxation of pBR322 substrate measured after 4 hrs by ... |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50515038

(CHEMBL4460653)Show SMILES Cc1c(CNC2CCC(CC2)NC(N)=N)cc(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 |(35.89,-12.49,;34.42,-12.01,;33.94,-10.55,;34.84,-9.3,;34.21,-7.9,;35.11,-6.65,;36.65,-6.82,;37.55,-5.58,;36.92,-4.17,;35.39,-4.01,;34.48,-5.26,;37.83,-2.92,;39.36,-3.08,;40.27,-1.84,;39.99,-4.49,;32.4,-10.56,;31.93,-12.02,;30.47,-12.5,;29.33,-11.47,;27.87,-11.95,;27.55,-13.46,;26.09,-13.94,;28.71,-14.49,;30.16,-14,;33.18,-12.92,;33.19,-14.46,;31.86,-15.23,;31.86,-16.77,;33.19,-17.54,;33.19,-19.08,;34.53,-16.77,;34.53,-15.22,)| Show InChI InChI=1S/C25H29Cl2N5/c1-16-18(15-30-21-8-10-22(11-9-21)31-25(28)29)14-24(17-2-4-19(26)5-3-17)32(16)23-12-6-20(27)7-13-23/h2-7,12-14,21-22,30H,8-11,15H2,1H3,(H4,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase S84L mutant |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50520948

(CHEMBL4443524)Show SMILES CNC(=O)c1c(oc2ccc(O)c(CN3CCCCC3)c12)-c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O4/c1-23-22(27)20-19-16(13-24-11-3-2-4-12-24)17(26)9-10-18(19)28-21(20)14-5-7-15(25)8-6-14/h5-10,25-26H,2-4,11-13H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50339000
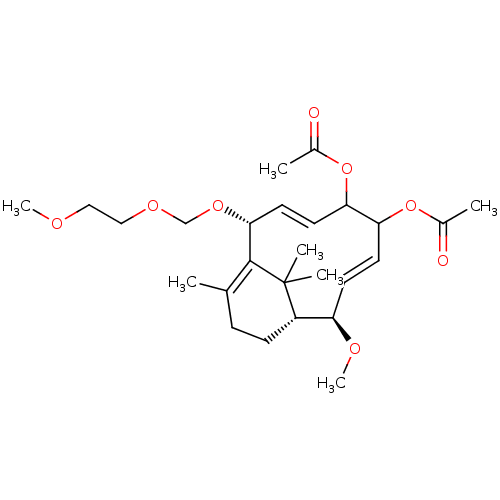
((1R,2R,9R)-2-methoxy-9-((2-methoxyethoxy)methoxy)-...)Show SMILES COCCOCO[C@@H]1\C=C/C(OC(C)=O)C(OC(C)=O)\C=C\[C@@H](OC)[C@@H]2CCC(C)=C1C2(C)C |r,c:8,t:20,29| Show InChI InChI=1S/C26H40O8/c1-17-8-9-20-21(30-7)10-11-22(33-18(2)27)23(34-19(3)28)12-13-24(25(17)26(20,4)5)32-16-31-15-14-29-6/h10-13,20-24H,8-9,14-16H2,1-7H3/b11-10+,13-12-/t20-,21+,22?,23?,24+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom... |
ACS Med Chem Lett 1: 416-421 (2010)
Article DOI: 10.1021/ml100118k
BindingDB Entry DOI: 10.7270/Q28K7B2N |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615162

(CHEMBL5286328)Show SMILES [H][C@]12C[C@]3([H])OC(C)(C)O[C@]3([H])[C@@H](O)[C@@]1([H])NC(=O)O2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50598092
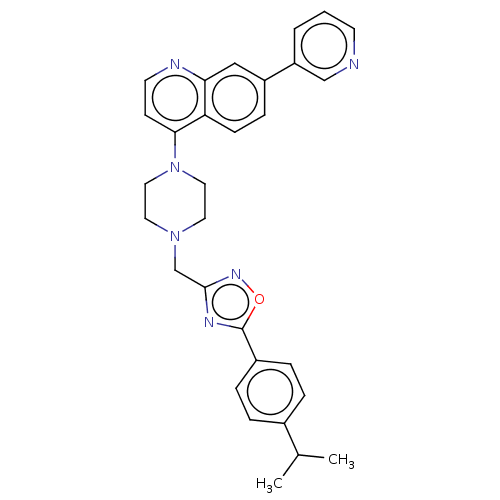
(CHEMBL4473719)Show SMILES CC(C)c1ccc(cc1)-c1nc(CN2CCN(CC2)c2ccnc3cc(ccc23)-c2cccnc2)no1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50583310
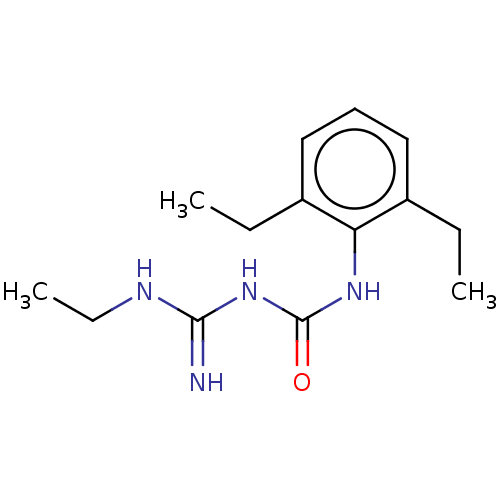
(CHEMBL5082711) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50517949

(CHEMBL4447540)Show InChI InChI=1S/C15H12N4O4S2/c1-2-23-15(22)19-11(20)8-4-6-24-13(8)18-12(21)14-17-9-7-16-5-3-10(9)25-14/h3-7H,2H2,1H3,(H,18,21)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583310

(CHEMBL5082711) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598091
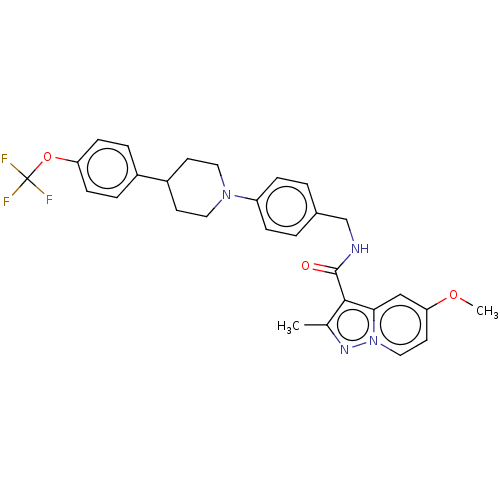
(CHEMBL3612958)Show SMILES COc1ccn2nc(C)c(C(=O)NCc3ccc(cc3)N3CCC(CC3)c3ccc(OC(F)(F)F)cc3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50615162

(CHEMBL5286328)Show SMILES [H][C@]12C[C@]3([H])OC(C)(C)O[C@]3([H])[C@@H](O)[C@@]1([H])NC(=O)O2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50515037

(CHEMBL4436044)Show SMILES CC(C)c1ccc(cc1)-n1c(C)c(CNC2CCCCCC2)cc1-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H35ClN2/c1-20(2)22-12-16-27(17-13-22)31-21(3)24(19-30-26-8-6-4-5-7-9-26)18-28(31)23-10-14-25(29)15-11-23/h10-18,20,26,30H,4-9,19H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase S84L mutant |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615162

(CHEMBL5286328)Show SMILES [H][C@]12C[C@]3([H])OC(C)(C)O[C@]3([H])[C@@H](O)[C@@]1([H])NC(=O)O2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598088
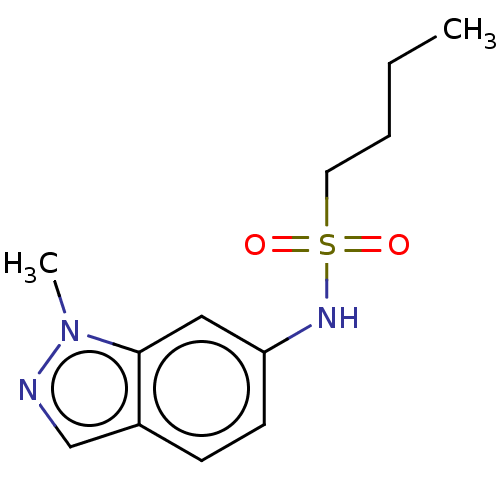
(CHEMBL2098221 | GSK3011724A | TCMDC-142399) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598087
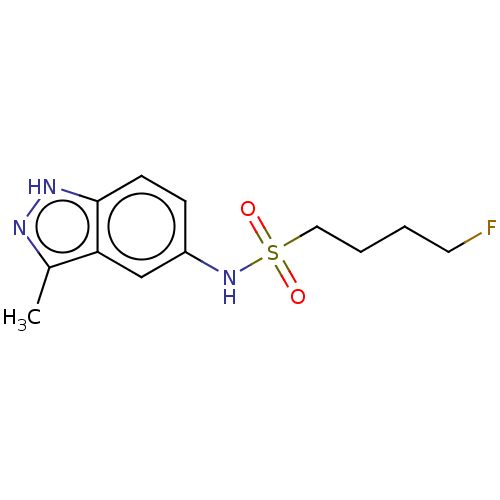
(CHEMBL5181555) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50598089
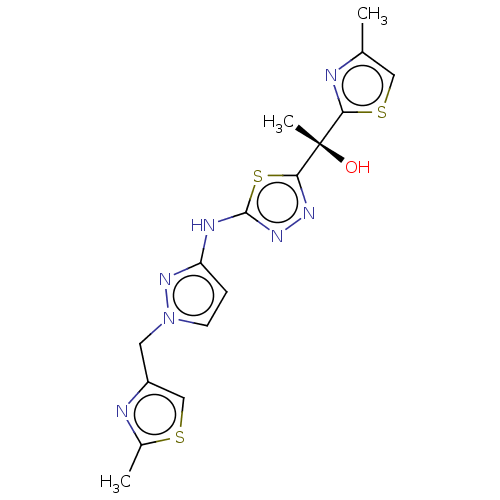
(CHEMBL5186224)Show SMILES Cc1csc(n1)[C@](C)(O)c1nnc(Nc2ccn(Cc3csc(C)n3)n2)s1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00227
BindingDB Entry DOI: 10.7270/Q2RR238M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50615162

(CHEMBL5286328)Show SMILES [H][C@]12C[C@]3([H])OC(C)(C)O[C@]3([H])[C@@H](O)[C@@]1([H])NC(=O)O2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50515038

(CHEMBL4460653)Show SMILES Cc1c(CNC2CCC(CC2)NC(N)=N)cc(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 |(35.89,-12.49,;34.42,-12.01,;33.94,-10.55,;34.84,-9.3,;34.21,-7.9,;35.11,-6.65,;36.65,-6.82,;37.55,-5.58,;36.92,-4.17,;35.39,-4.01,;34.48,-5.26,;37.83,-2.92,;39.36,-3.08,;40.27,-1.84,;39.99,-4.49,;32.4,-10.56,;31.93,-12.02,;30.47,-12.5,;29.33,-11.47,;27.87,-11.95,;27.55,-13.46,;26.09,-13.94,;28.71,-14.49,;30.16,-14,;33.18,-12.92,;33.19,-14.46,;31.86,-15.23,;31.86,-16.77,;33.19,-17.54,;33.19,-19.08,;34.53,-16.77,;34.53,-15.22,)| Show InChI InChI=1S/C25H29Cl2N5/c1-16-18(15-30-21-8-10-22(11-9-21)31-25(28)29)14-24(17-2-4-19(26)5-3-17)32(16)23-12-6-20(27)7-13-23/h2-7,12-14,21-22,30H,8-11,15H2,1H3,(H4,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus topoisomerase-4 expressed in Escherichia coli assessed as relaxation of pBR322 substrate measured after 4 hrs by ... |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50515039

(CHEMBL4530791)Show SMILES CC(C)c1ccc(cc1)-c1cc(CNC2CCCCC2)c(C)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN2/c1-19(2)21-9-11-22(12-10-21)27-17-23(18-29-25-7-5-4-6-8-25)20(3)30(27)26-15-13-24(28)14-16-26/h9-17,19,25,29H,4-8,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase S84L mutant |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase S84L mutant |
Eur J Med Chem 178: 500-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.087
BindingDB Entry DOI: 10.7270/Q26Q21KH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data