

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50606398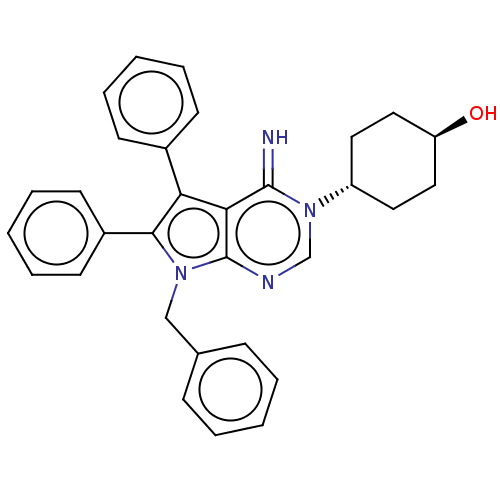 (ML-246 | Metarrestin | Ml-246) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00204 BindingDB Entry DOI: 10.7270/Q2FN1B9R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50606398 (ML-246 | Metarrestin | Ml-246) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00204 BindingDB Entry DOI: 10.7270/Q2FN1B9R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50606398 (ML-246 | Metarrestin | Ml-246) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00204 BindingDB Entry DOI: 10.7270/Q2FN1B9R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50606398 (ML-246 | Metarrestin | Ml-246) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00204 BindingDB Entry DOI: 10.7270/Q2FN1B9R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50606398 (ML-246 | Metarrestin | Ml-246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00204 BindingDB Entry DOI: 10.7270/Q2FN1B9R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331863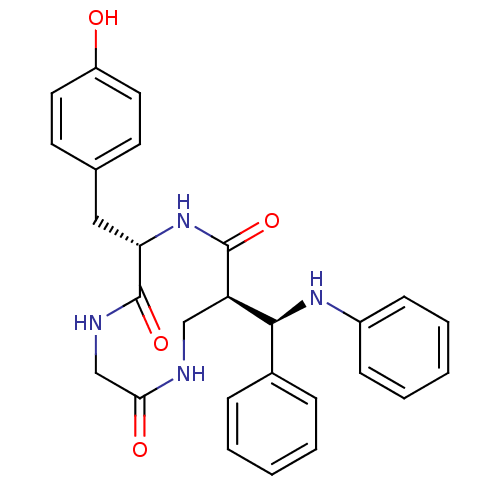 (6-(4-Hydroxy-benzyl)-9-(phenyl-phenylamino-methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50345500 (6-oxo-1,2,9,10-tetradehydro-3,4,5,6-tetrahydro-5-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
India Institute of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 19: 3274-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.024 BindingDB Entry DOI: 10.7270/Q2XP759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331859 (4,9-Dioxo-3-(phenyl-phenylamino-methyl)-[1,5]diazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331860 (9-(Phenyl-phenylamino-methyl)-[1,4,7]triazecane-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50345501 (CHEMBL1784317 | rac-6-oxo-1,2,3,4,5,6,7,8,9,10-dec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
India Institute of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 19: 3274-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.024 BindingDB Entry DOI: 10.7270/Q2XP759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331858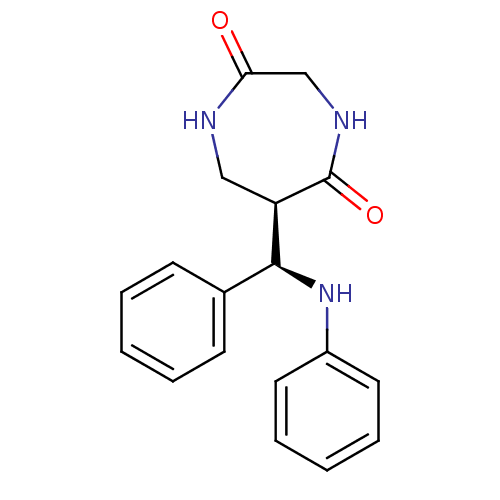 (6-(Phenyl-phenylamino-methyl)-[1,4]diazepane-2,5-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331864 (3,6,9-Trimethyl-12-(phenyl-phenylamino-methyl)-1,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331861 (9-(Furan-2-yl-phenylamino-methyl)-[1,4,7]triazecan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50331862 (3,6-Dimethyl-9-(phenyl-phenylamino-methyl)-[1,4,7]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 8365-73 (2010) Article DOI: 10.1016/j.bmc.2010.09.052 BindingDB Entry DOI: 10.7270/Q28S4Q5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206892 (CHEMBL3948489) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206895 (CHEMBL3983017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206890 (CHEMBL3910830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206891 (CHEMBL3921785) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206897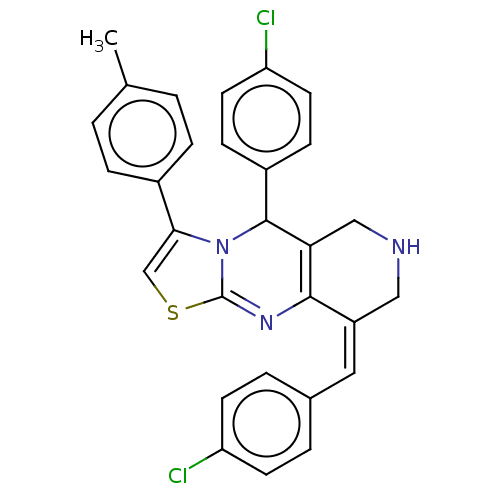 (CHEMBL3945423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206885 (CHEMBL3930722) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206896 (CHEMBL3958208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206889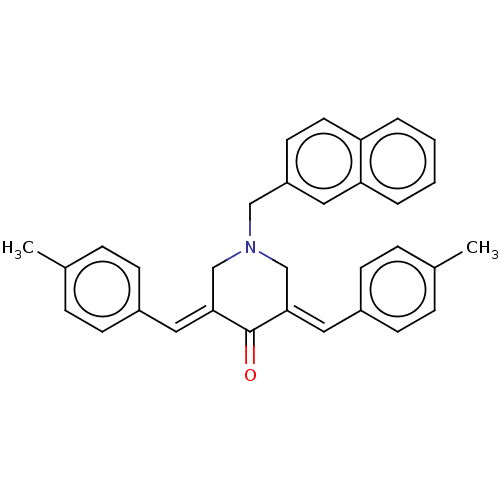 (CHEMBL3928840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206893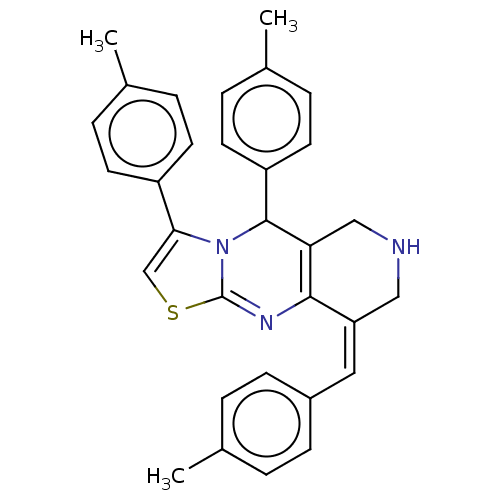 (CHEMBL3901794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206886 (CHEMBL3955393) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206894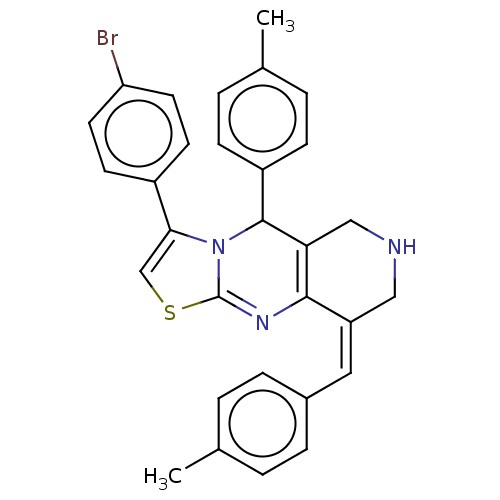 (CHEMBL3972865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206887 (CHEMBL3976003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206888 (CHEMBL3984034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50345500 (6-oxo-1,2,9,10-tetradehydro-3,4,5,6-tetrahydro-5-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Institute of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 19: 3274-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.024 BindingDB Entry DOI: 10.7270/Q2XP759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50345501 (CHEMBL1784317 | rac-6-oxo-1,2,3,4,5,6,7,8,9,10-dec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Institute of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 19: 3274-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.024 BindingDB Entry DOI: 10.7270/Q2XP759N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||