

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415448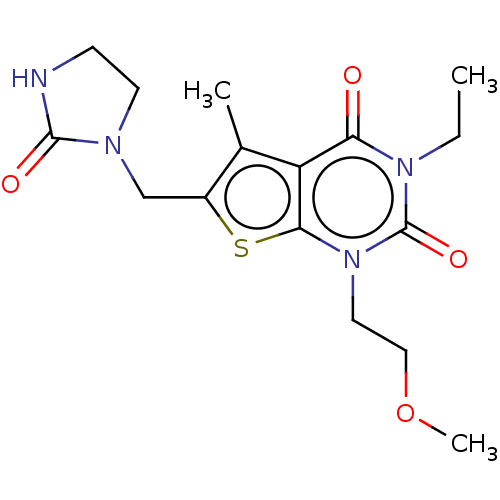 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415450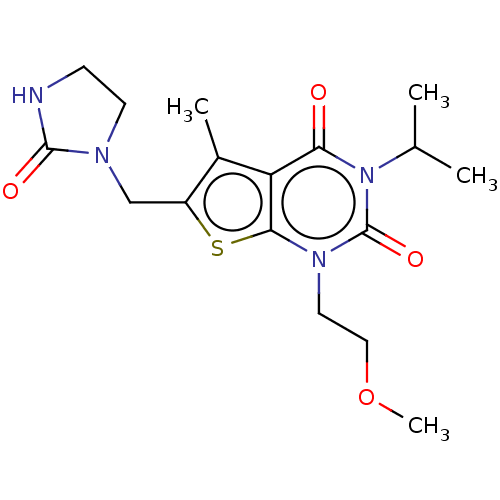 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415451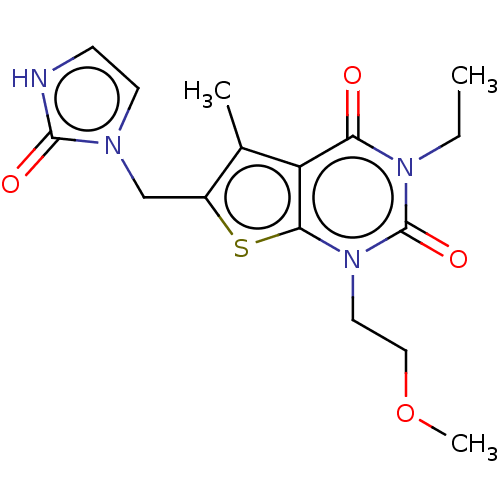 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415449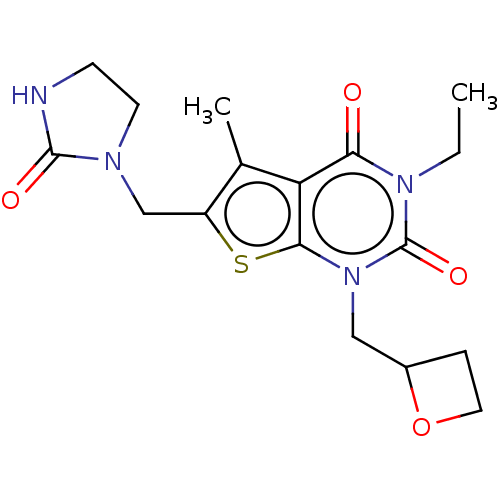 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415452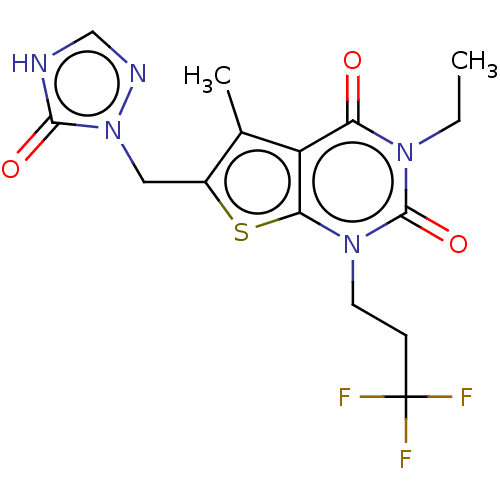 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415453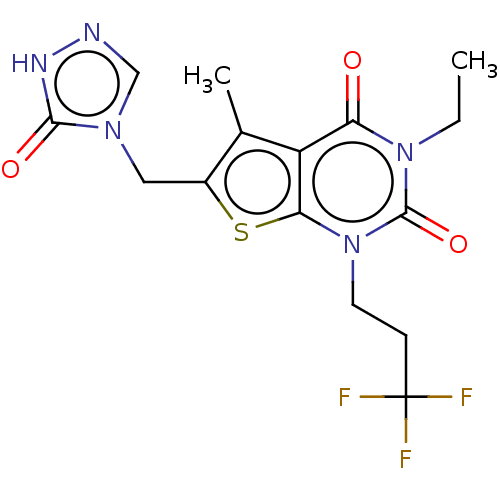 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415452 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415452 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228279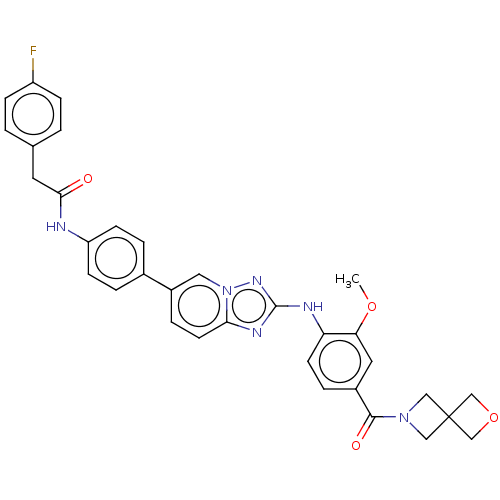 (US9555022, Example 02.17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228278 (US9555022, Example 02.16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236684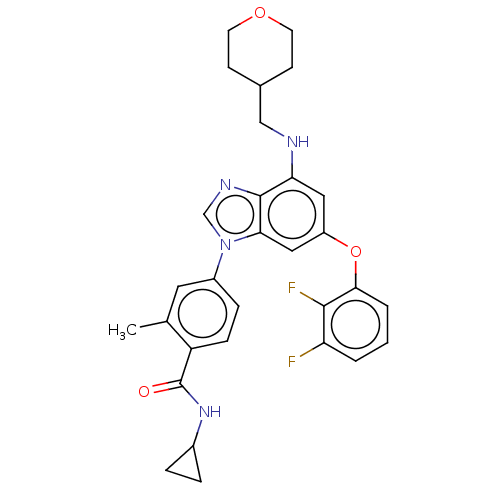 (US9388140, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228270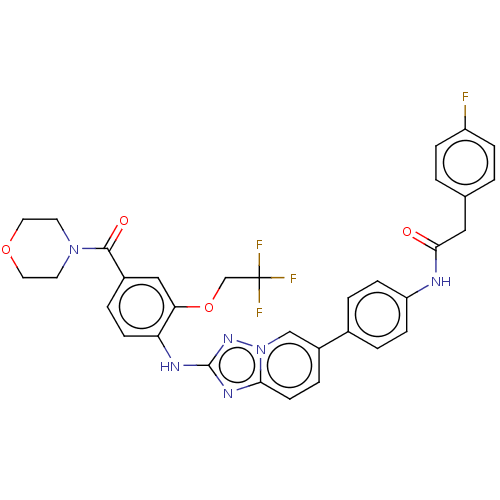 (US9555022, Example 02.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236661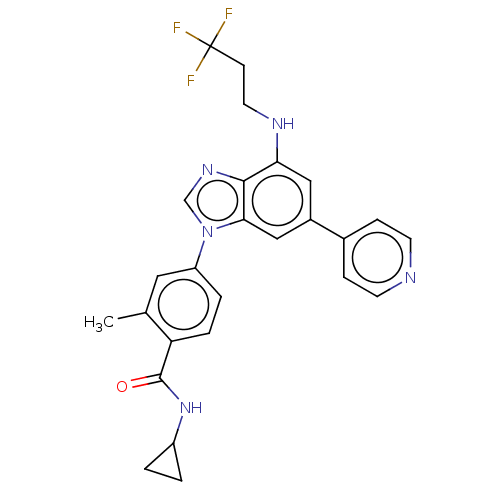 (US9388140, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581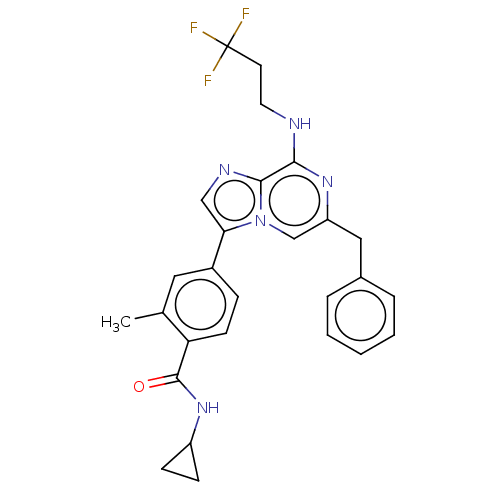 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236713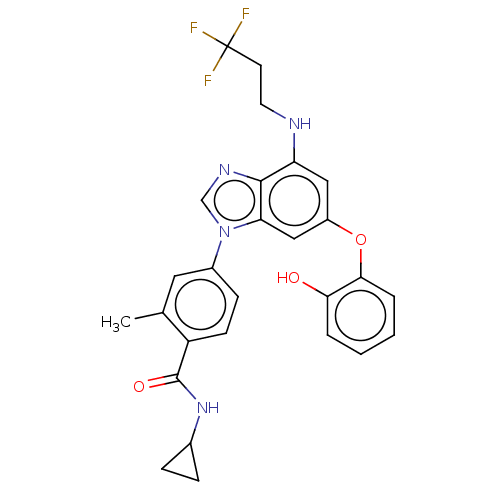 (US9388140, 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM258444 (US9512130, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236715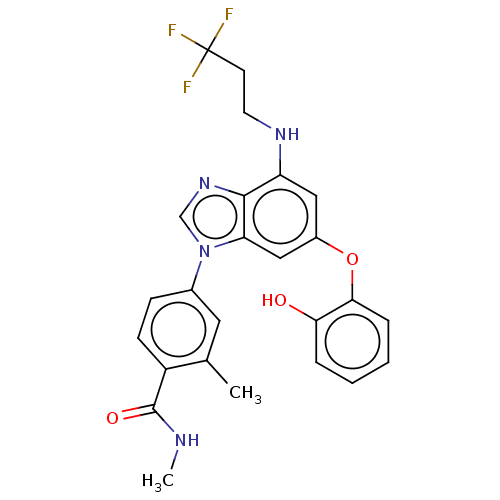 (US9388140, 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236683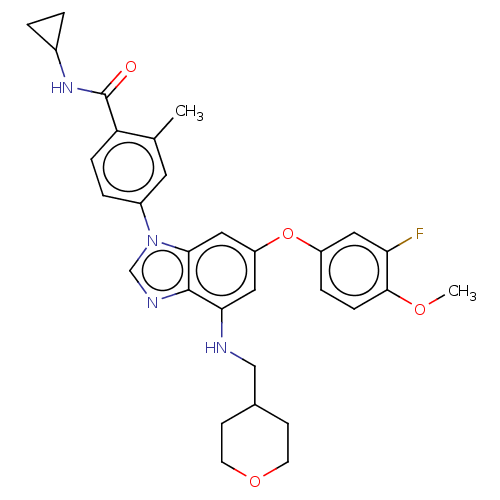 (BDBM236703 | US9388140, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236673 (BDBM236695 | US9388140, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236747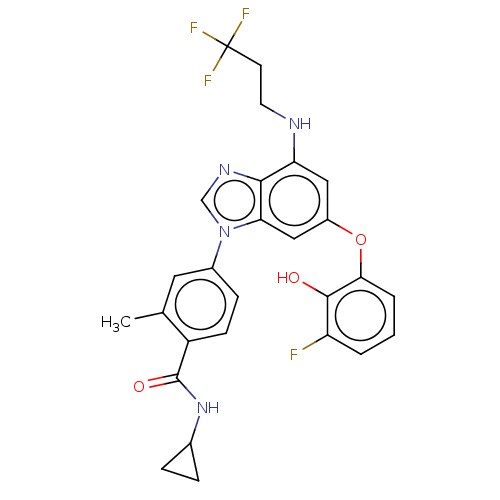 (US9388140, 91) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236697 (US9388140, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236668 (US9388140, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542583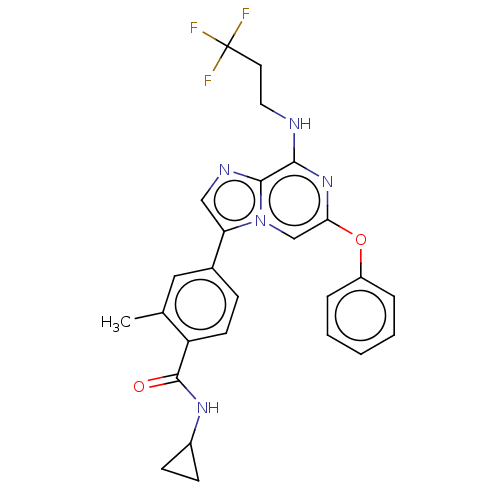 (CHEMBL4634949) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236673 (BDBM236695 | US9388140, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM329340 ((2R)—N-{4-[2-({4-[(3-fluoroazetidin-1-yl)carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236696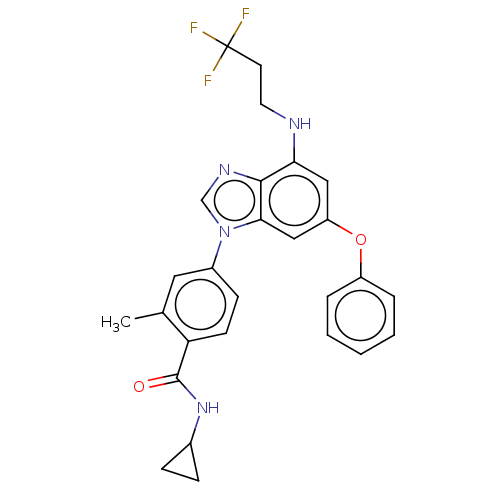 (US9388140, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218770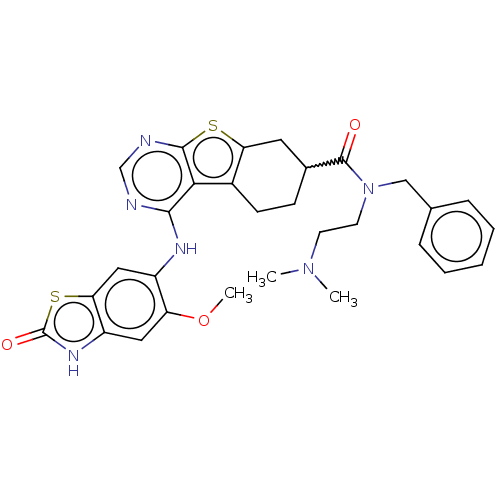 (US9296757, 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM236766 (US9388140, 111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.7 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9388140 (2016) BindingDB Entry DOI: 10.7270/Q2BP01QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1270 total ) | Next | Last >> |