 Found 1290 hits with Last Name = 'holson' and Initial = 'e'
Found 1290 hits with Last Name = 'holson' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition full length human recombinant HDAC2 expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM178095
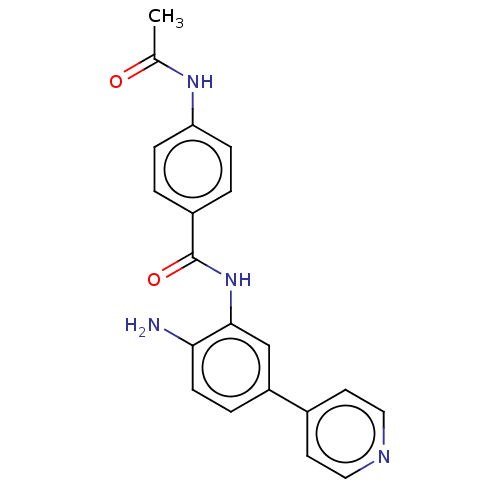
(BRD2492)Show SMILES CC(=O)Nc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1ccncc1 Show InChI InChI=1S/C20H18N4O2/c1-13(25)23-17-5-2-15(3-6-17)20(26)24-19-12-16(4-7-18(19)21)14-8-10-22-11-9-14/h2-12H,21H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM178095

(BRD2492)Show SMILES CC(=O)Nc1ccc(cc1)C(=O)Nc1cc(ccc1N)-c1ccncc1 Show InChI InChI=1S/C20H18N4O2/c1-13(25)23-17-5-2-15(3-6-17)20(26)24-19-12-16(4-7-18(19)21)14-8-10-22-11-9-14/h2-12H,21H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.8 | 19 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | -43.4 | 46 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM178100
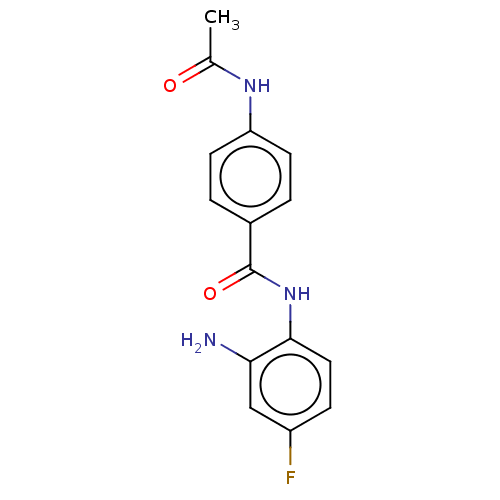
(BRD3308 | US11377423, Cmpd 1)Show InChI InChI=1S/C15H14FN3O2/c1-9(20)18-12-5-2-10(3-6-12)15(21)19-14-7-4-11(16)8-13(14)17/h2-8H,17H2,1H3,(H,18,20)(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | -43.0 | 64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457880

(US10752588, Compound 94a)Show SMILES Fc1ccccc1OCCN1CCC(CC1)C(=O)N[C@@H](c1ccc2OC(F)(F)Oc2c1)c1ccccn1 |r| Show InChI InChI=1S/C27H26F3N3O4/c28-20-5-1-2-7-22(20)35-16-15-33-13-10-18(11-14-33)26(34)32-25(21-6-3-4-12-31-21)19-8-9-23-24(17-19)37-27(29,30)36-23/h1-9,12,17-18,25H,10-11,13-16H2,(H,32,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457876
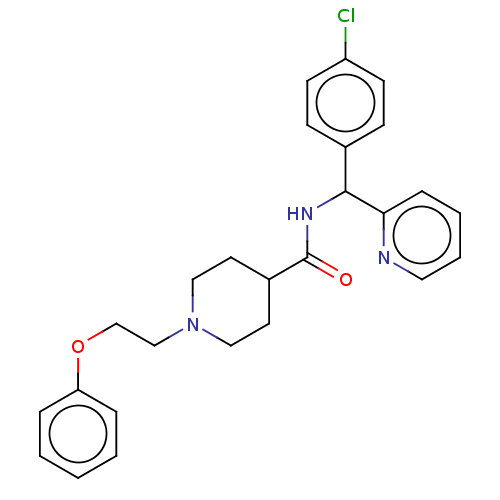
(US10752588, Compound 63)Show SMILES Clc1ccc(cc1)C(NC(=O)C1CCN(CCOc2ccccc2)CC1)c1ccccn1 Show InChI InChI=1S/C26H28ClN3O2/c27-22-11-9-20(10-12-22)25(24-8-4-5-15-28-24)29-26(31)21-13-16-30(17-14-21)18-19-32-23-6-2-1-3-7-23/h1-12,15,21,25H,13-14,16-19H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457879

(US10752588, Compound 95)Show SMILES Fc1ccccc1OCCN1CCC(CC1)C(=O)NC(c1ccc2OCOc2c1)c1ccccn1 Show InChI InChI=1S/C27H28FN3O4/c28-21-5-1-2-7-23(21)33-16-15-31-13-10-19(11-14-31)27(32)30-26(22-6-3-4-12-29-22)20-8-9-24-25(17-20)35-18-34-24/h1-9,12,17,19,26H,10-11,13-16,18H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457899

(US10752588, Compound 125)Show SMILES FC(F)(F)CN(CC1CCN(CCOc2ccccc2)CC1)C(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C28H31ClF3N3O/c29-24-11-9-23(10-12-24)27(26-8-4-5-15-33-26)35(21-28(30,31)32)20-22-13-16-34(17-14-22)18-19-36-25-6-2-1-3-7-25/h1-12,15,22,27H,13-14,16-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457881
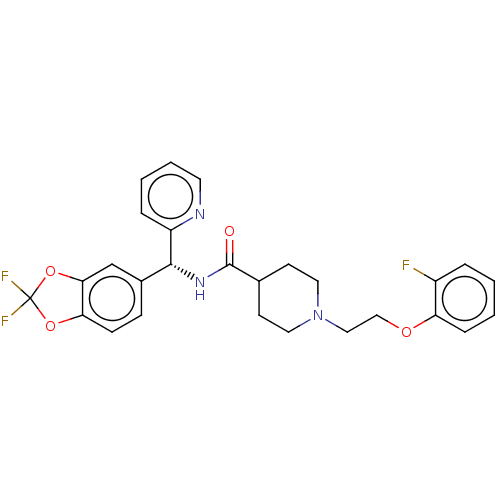
(US10752588, Compound 94b)Show SMILES Fc1ccccc1OCCN1CCC(CC1)C(=O)N[C@H](c1ccc2OC(F)(F)Oc2c1)c1ccccn1 |r| Show InChI InChI=1S/C27H26F3N3O4/c28-20-5-1-2-7-22(20)35-16-15-33-13-10-18(11-14-33)26(34)32-25(21-6-3-4-12-31-21)19-8-9-23-24(17-19)37-27(29,30)36-23/h1-9,12,17-18,25H,10-11,13-16H2,(H,32,34)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457883
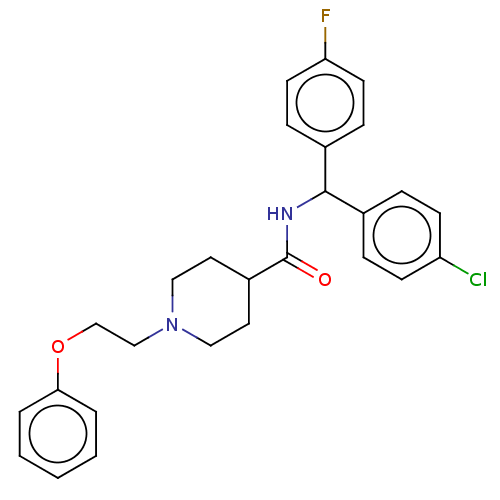
(US10752588, Compound 42)Show SMILES Fc1ccc(cc1)C(NC(=O)C1CCN(CCOc2ccccc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H28ClFN2O2/c28-23-10-6-20(7-11-23)26(21-8-12-24(29)13-9-21)30-27(32)22-14-16-31(17-15-22)18-19-33-25-4-2-1-3-5-25/h1-13,22,26H,14-19H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457884

(US10752588, Compound 79)Show SMILES CN(CC1CCN(CCOc2ccccc2)CC1)C(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C27H32ClN3O/c1-30(27(26-9-5-6-16-29-26)23-10-12-24(28)13-11-23)21-22-14-17-31(18-15-22)19-20-32-25-7-3-2-4-8-25/h2-13,16,22,27H,14-15,17-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457885

(US10752588, Compound 79b)Show SMILES CN(CC1CCN(CCOc2ccccc2)CC1)[C@H](c1ccc(Cl)cc1)c1ccccn1 |r| Show InChI InChI=1S/C27H32ClN3O/c1-30(27(26-9-5-6-16-29-26)23-10-12-24(28)13-11-23)21-22-14-17-31(18-15-22)19-20-32-25-7-3-2-4-8-25/h2-13,16,22,27H,14-15,17-21H2,1H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457886

(US10752588, Compound 114)Show SMILES Clc1ccc(cc1)C(NCC1CCN(CCOc2ccccc2)CC1)c1ccccn1 Show InChI InChI=1S/C26H30ClN3O/c27-23-11-9-22(10-12-23)26(25-8-4-5-15-28-25)29-20-21-13-16-30(17-14-21)18-19-31-24-6-2-1-3-7-24/h1-12,15,21,26,29H,13-14,16-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457887

(US10752588, Compound 114a)Show SMILES Clc1ccc(cc1)[C@@H](NCC1CCN(CCOc2ccccc2)CC1)c1ccccn1 |r| Show InChI InChI=1S/C26H30ClN3O/c27-23-11-9-22(10-12-23)26(25-8-4-5-15-28-25)29-20-21-13-16-30(17-14-21)18-19-31-24-6-2-1-3-7-24/h1-12,15,21,26,29H,13-14,16-20H2/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457889

(US10752588, Compound 72 | US10752588, Compound 72a)Show SMILES CN(CC1CCN(CCOc2ccccc2F)CC1)C(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C27H31ClFN3O/c1-31(27(25-7-4-5-15-30-25)22-9-11-23(28)12-10-22)20-21-13-16-32(17-14-21)18-19-33-26-8-3-2-6-24(26)29/h2-12,15,21,27H,13-14,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457891

(US10752588, Compound 77a)Show SMILES COc1cccc(c1)C(N(C)CC1CCN(CCOc2ccccc2F)CC1)c1ccccn1 Show InChI InChI=1S/C28H34FN3O2/c1-31(28(26-11-5-6-15-30-26)23-8-7-9-24(20-23)33-2)21-22-13-16-32(17-14-22)18-19-34-27-12-4-3-10-25(27)29/h3-12,15,20,22,28H,13-14,16-19,21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457892

(US10752588, Compound 66)Show SMILES Clc1ccc(cc1)C(NC(=O)C1CCN(CCOc2ccccc2Cl)CC1)c1ccccn1 Show InChI InChI=1S/C26H27Cl2N3O2/c27-21-10-8-19(9-11-21)25(23-6-3-4-14-29-23)30-26(32)20-12-15-31(16-13-20)17-18-33-24-7-2-1-5-22(24)28/h1-11,14,20,25H,12-13,15-18H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457894
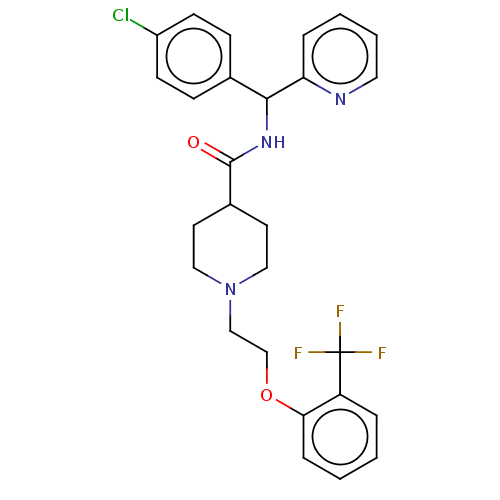
(US10752588, Compound 6)Show SMILES FC(F)(F)c1ccccc1OCCN1CCC(CC1)C(=O)NC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C27H27ClF3N3O2/c28-21-10-8-19(9-11-21)25(23-6-3-4-14-32-23)33-26(35)20-12-15-34(16-13-20)17-18-36-24-7-2-1-5-22(24)27(29,30)31/h1-11,14,20,25H,12-13,15-18H2,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457896
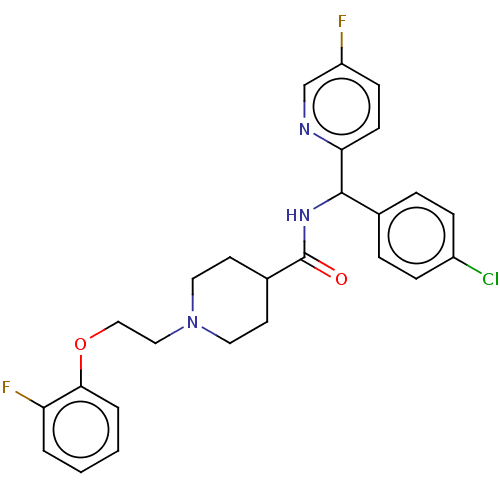
(US10752588, Compound 92)Show SMILES Fc1ccc(nc1)C(NC(=O)C1CCN(CCOc2ccccc2F)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H26ClF2N3O2/c27-20-7-5-18(6-8-20)25(23-10-9-21(28)17-30-23)31-26(33)19-11-13-32(14-12-19)15-16-34-24-4-2-1-3-22(24)29/h1-10,17,19,25H,11-16H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457897

(US10752588, Compound 53)Show SMILES Fc1ccc(cc1)C(NC(=O)C1CCN(CCOc2ccccc2Cl)CC1)c1ccccn1 Show InChI InChI=1S/C26H27ClFN3O2/c27-22-5-1-2-7-24(22)33-18-17-31-15-12-20(13-16-31)26(32)30-25(23-6-3-4-14-29-23)19-8-10-21(28)11-9-19/h1-11,14,20,25H,12-13,15-18H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457898
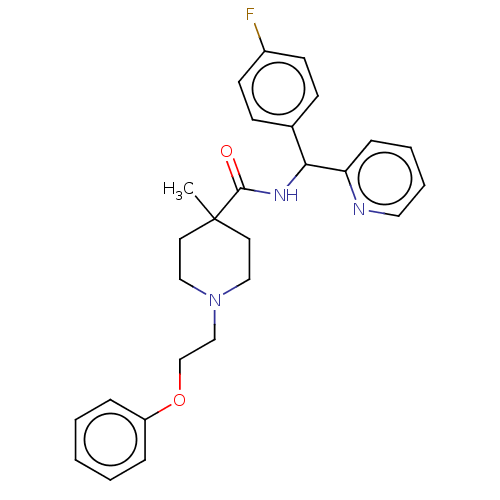
(US10752588, Compound 69b)Show SMILES CC1(CCN(CCOc2ccccc2)CC1)C(=O)NC(c1ccc(F)cc1)c1ccccn1 Show InChI InChI=1S/C27H30FN3O2/c1-27(14-17-31(18-15-27)19-20-33-23-7-3-2-4-8-23)26(32)30-25(24-9-5-6-16-29-24)21-10-12-22(28)13-11-21/h2-13,16,25H,14-15,17-20H2,1H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50189903
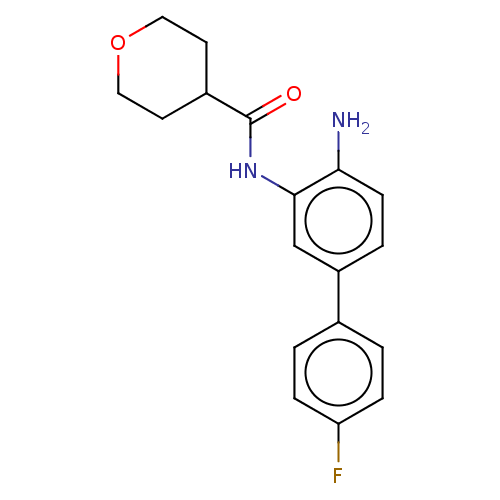
(CHEMBL3828396 | US11572368, Compound 54)Show InChI InChI=1S/C18H19FN2O2/c19-15-4-1-12(2-5-15)14-3-6-16(20)17(11-14)21-18(22)13-7-9-23-10-8-13/h1-6,11,13H,7-10,20H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580141

(US11498896, Compound 106)Show SMILES CC1(CCN(CCOc2ccccc2C(F)(F)F)CC1)C(=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580142
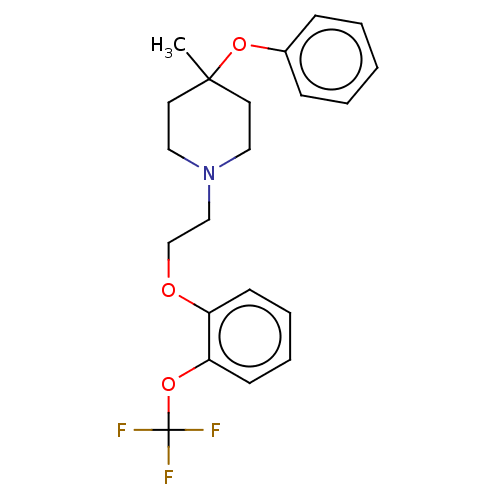
(US11498896, Compound 110) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580146
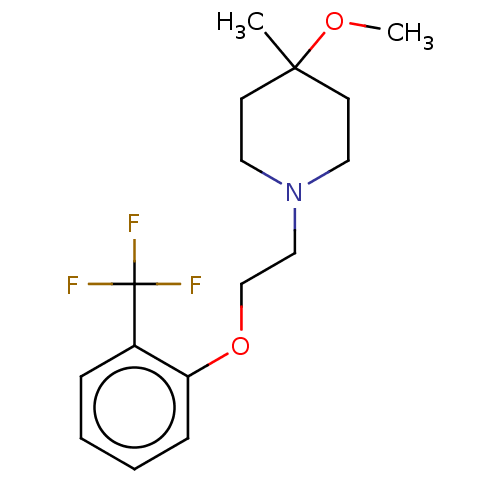
(US11498896, Compound 123) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580148
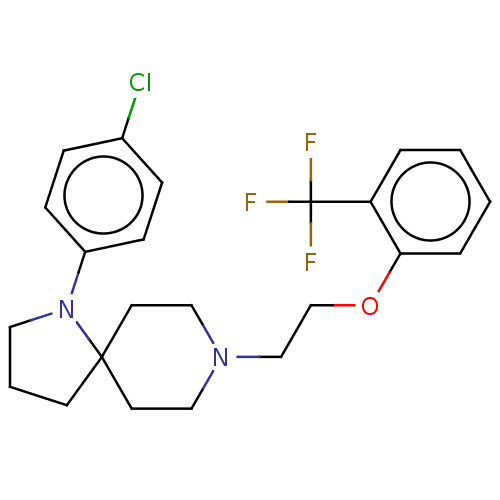
(US11498896, Compound 135)Show SMILES FC(F)(F)c1ccccc1OCCN1CCC2(CCCN2c2ccc(Cl)cc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580149

(US11498896, Compound 137) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580162

(US11498896, Compound 211)Show SMILES CC(=O)NC1(C)CCN(C[C@H]2COc3ccc(Br)cc3O2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457888

(US10752588, Compound 105)Show SMILES Clc1ccc(cc1)C(N1CCC2(C1)CCN(CCOc1ccccc1)CC2)c1ccccn1 Show InChI InChI=1S/C28H32ClN3O/c29-24-11-9-23(10-12-24)27(26-8-4-5-16-30-26)32-19-15-28(22-32)13-17-31(18-14-28)20-21-33-25-6-2-1-3-7-25/h1-12,16,27H,13-15,17-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457889

(US10752588, Compound 72 | US10752588, Compound 72a)Show SMILES CN(CC1CCN(CCOc2ccccc2F)CC1)C(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C27H31ClFN3O/c1-31(27(25-7-4-5-15-30-25)22-9-11-23(28)12-10-22)20-21-13-16-32(17-14-21)18-19-33-26-8-3-2-6-24(26)29/h2-12,15,21,27H,13-14,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM457901

(US10752588, Compound 144a)Show SMILES CC1(CCN(CCOc2ccccc2)CC1)C(=O)NC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C27H30ClN3O2/c1-27(14-17-31(18-15-27)19-20-33-23-7-3-2-4-8-23)26(32)30-25(24-9-5-6-16-29-24)21-10-12-22(28)13-11-21/h2-13,16,25H,14-15,17-20H2,1H3,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Massachusetts Institute of Technology
US Patent
| Assay Description
radioligand binding assay. |
US Patent US10752588 (2020)
BindingDB Entry DOI: 10.7270/Q27H1NNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580132

(US11498896, Compound 86) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580136

(US11498896, Compound 98a) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50189911
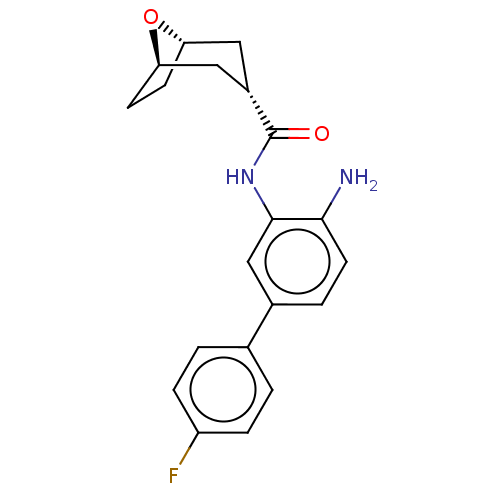
(CHEMBL3827611)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)C(=O)Nc1cc(ccc1N)-c1ccc(F)cc1)O2 |r| Show InChI InChI=1S/C20H21FN2O2/c21-15-4-1-12(2-5-15)13-3-8-18(22)19(11-13)23-20(24)14-9-16-6-7-17(10-14)25-16/h1-5,8,11,14,16-17H,6-7,9-10,22H2,(H,23,24)/t14-,16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 223 | -38.0 | 147 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | -36.0 | 398 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition full length human recombinant HDAC3 expressed in baculovirus using carboxyfluorescein (FAM)-labeled acetylated/ trifluoroacetylated peptid... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50189911

(CHEMBL3827611)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)C(=O)Nc1cc(ccc1N)-c1ccc(F)cc1)O2 |r| Show InChI InChI=1S/C20H21FN2O2/c21-15-4-1-12(2-5-15)13-3-8-18(22)19(11-13)23-20(24)14-9-16-6-7-17(10-14)25-16/h1-5,8,11,14,16-17H,6-7,9-10,22H2,(H,23,24)/t14-,16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 519 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition full length human recombinant HDAC2 expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580152

(US11498896, Compound 157)Show SMILES CC1(CCN(CCOc2ccc(F)cc2C(F)(F)F)CC1)Oc1ccc(Cl)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580155

(US11498896, Compound 204b) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580157

(US11498896, Compound 205) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM580158

(US11498896, Compound 206)Show SMILES CC1(CCN(C[C@H]2COc3ccccc3O2)CC1)C(=O)N1CCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The D2 binding Ki values were determined using a radioligand binding assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28919P8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data