

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50532693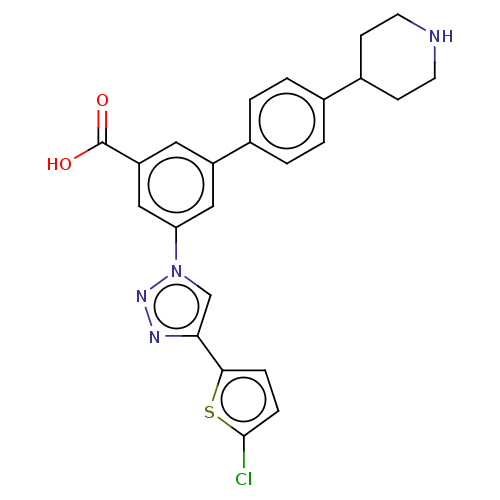 (CHEMBL4544251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50532691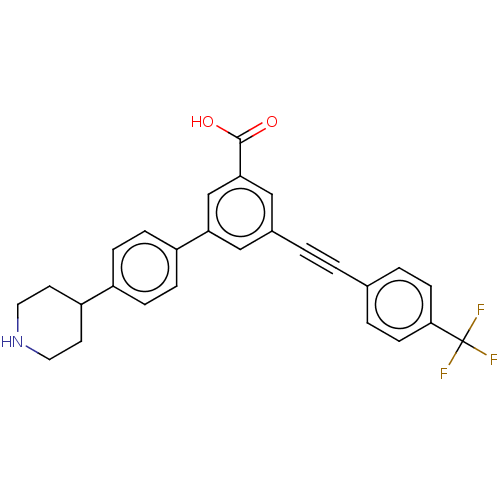 (CHEMBL4455037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to guinea pig sigma1 receptor by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50532691 (CHEMBL4455037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to guinea pig sigma1 receptor by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278487 (CHEMBL3585376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of 125-I echistatin from Vitronectin receptor (alpha v beta3) | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50532691 (CHEMBL4455037) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50532691 (CHEMBL4455037) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278488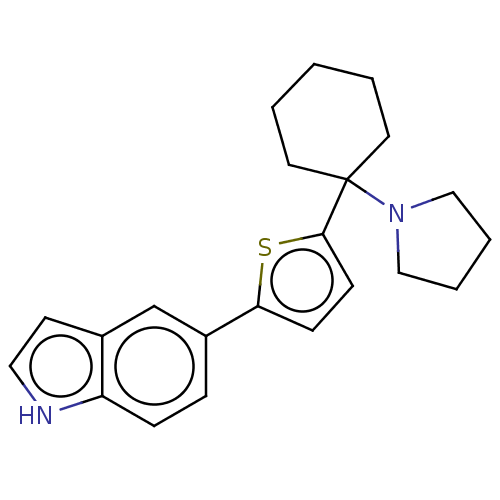 (CHEMBL4159241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278486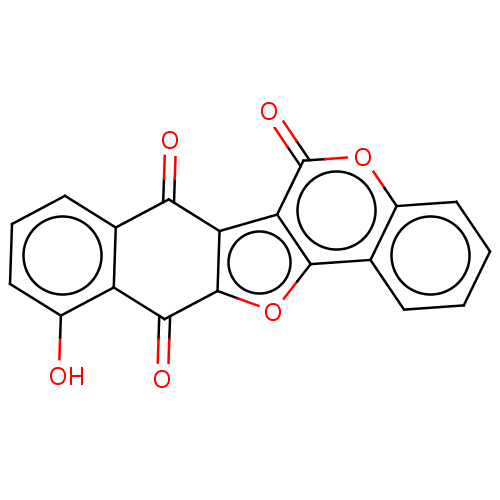 (CHEMBL4160100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed-type inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione d... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM77970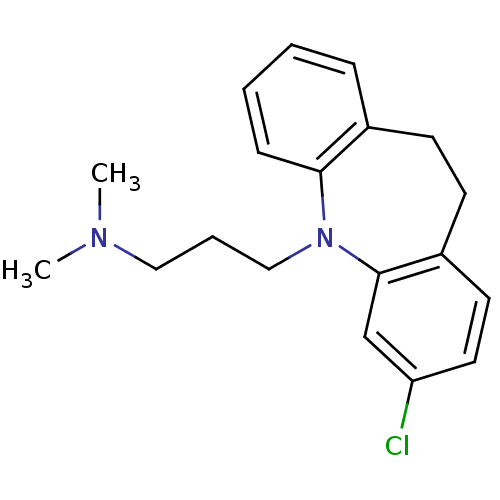 (3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50456168 (CHEMBL1800685) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50456168 (CHEMBL1800685) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278489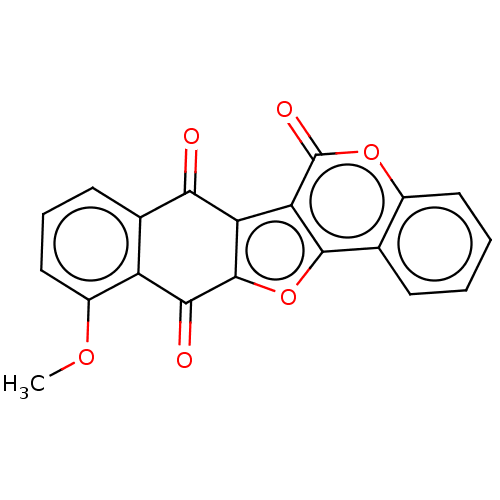 (CHEMBL4170316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Non-competitive inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothi... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523394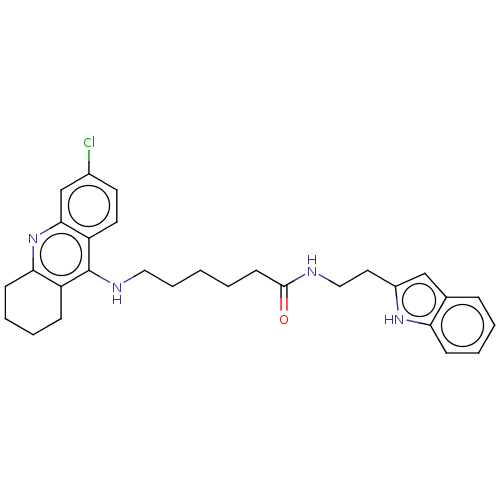 (CHEMBL4448188) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231951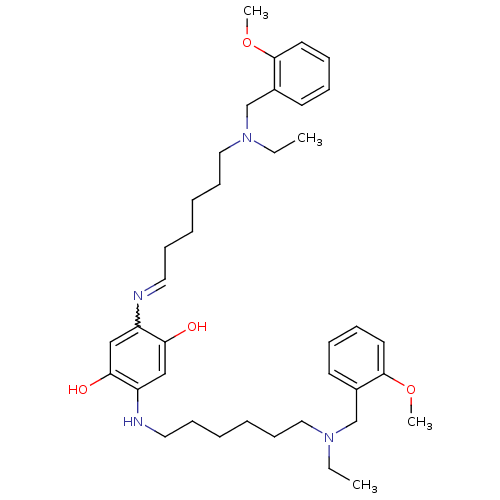 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028681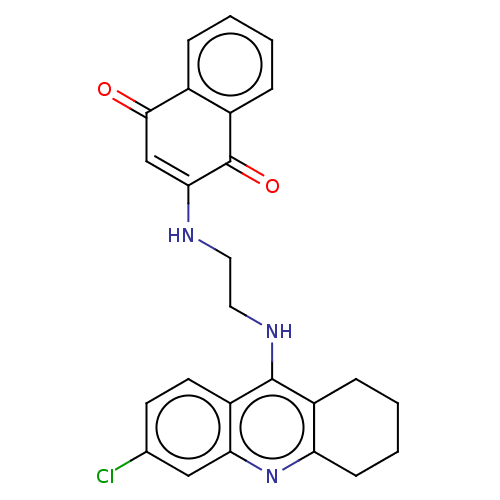 (CHEMBL3356532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028691 (CHEMBL3356951) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099602 (CHEMBL3343931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028682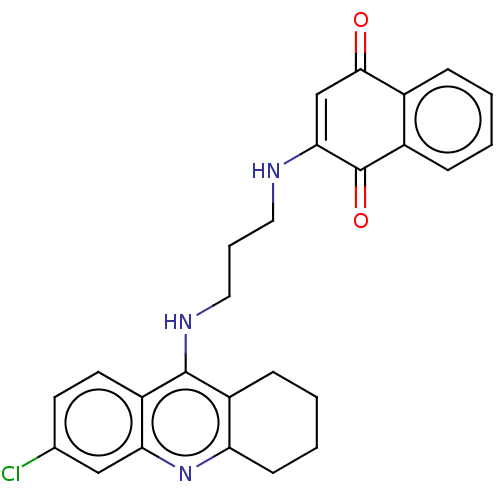 (CHEMBL3356533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028679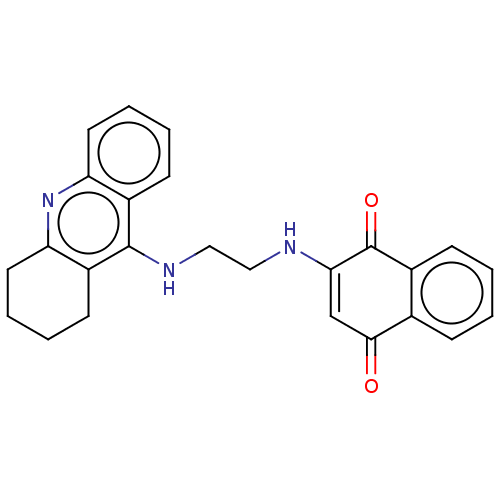 (CHEMBL3356530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523391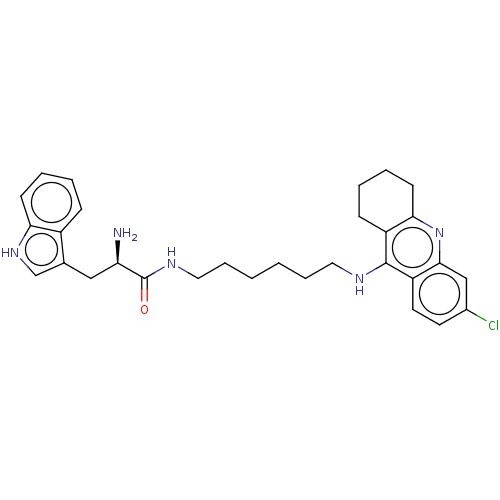 (CHEMBL4449083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523377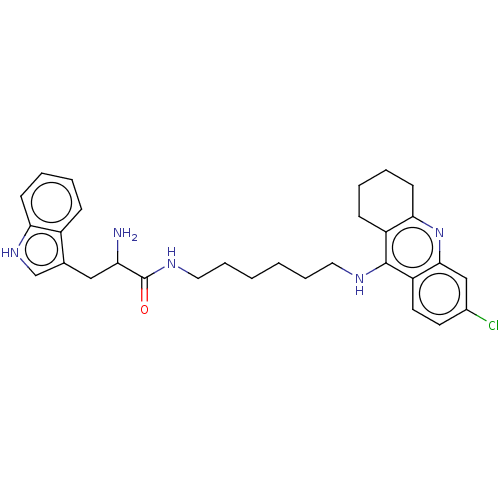 (CHEMBL4559593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028692 (CHEMBL3356952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028686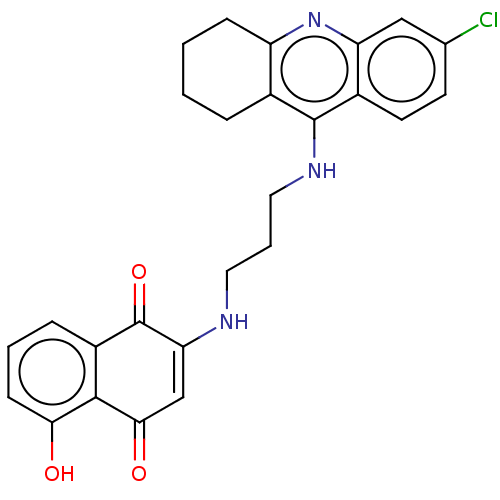 (CHEMBL3356537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523377 (CHEMBL4559593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028679 (CHEMBL3356530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028684 (CHEMBL3356535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028680 (CHEMBL3356531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523375 (CHEMBL4555818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523388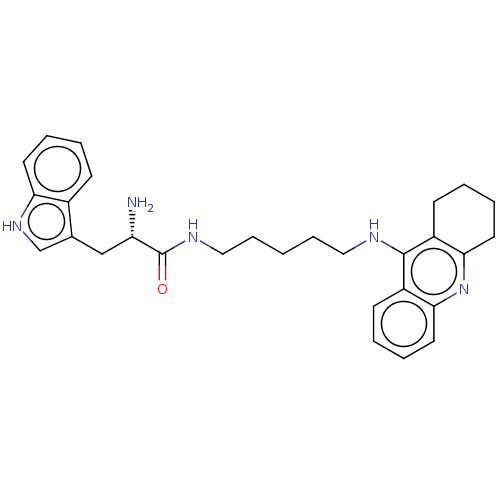 (CHEMBL4556575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 202 total ) | Next | Last >> |