

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50094670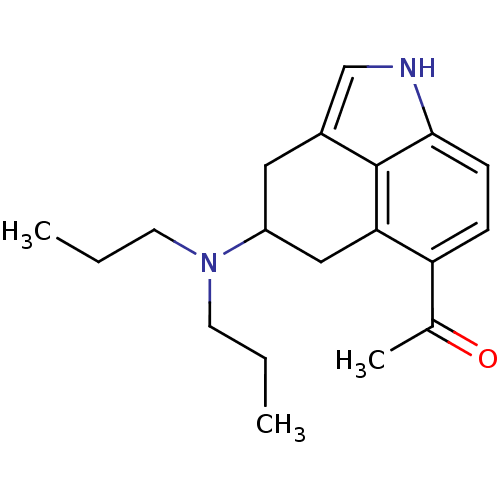 (1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition constant of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094673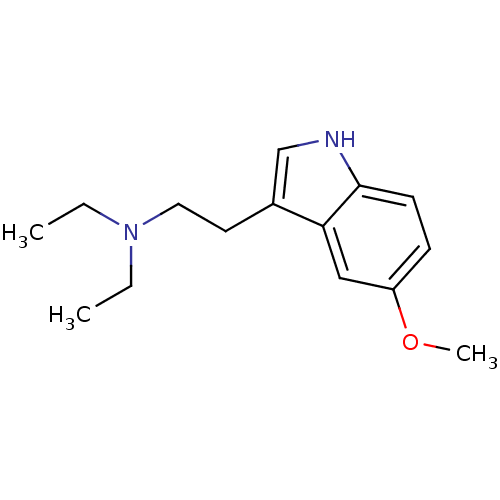 (CHEMBL342986 | Diethyl-[2-(5-methoxy-1H-indol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094677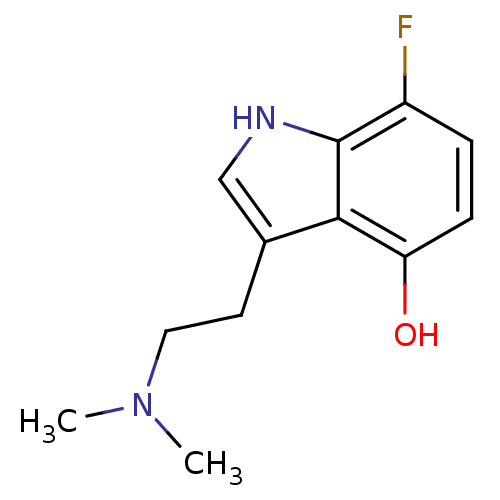 (3-(2-Dimethylamino-ethyl)-7-fluoro-1H-indol-4-ol |...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094671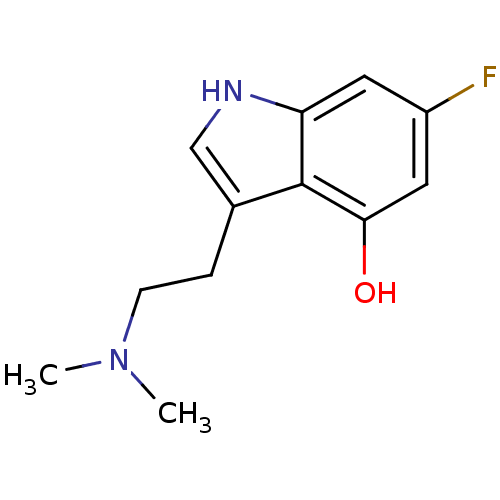 (3-(2-Dimethylamino-ethyl)-6-fluoro-1H-indol-4-ol |...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094675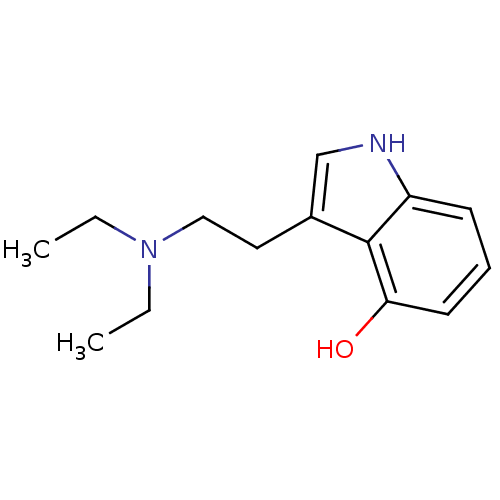 (3-(2-Diethylamino-ethyl)-1H-indol-4-ol | CHEMBL143...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094677 (3-(2-Dimethylamino-ethyl)-7-fluoro-1H-indol-4-ol |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094673 (CHEMBL342986 | Diethyl-[2-(5-methoxy-1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094669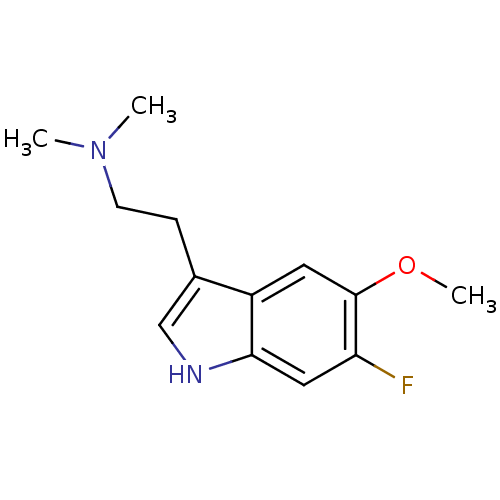 (CHEMBL337590 | [2-(6-Fluoro-5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094675 (3-(2-Diethylamino-ethyl)-1H-indol-4-ol | CHEMBL143...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094669 (CHEMBL337590 | [2-(6-Fluoro-5-methoxy-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094673 (CHEMBL342986 | Diethyl-[2-(5-methoxy-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094676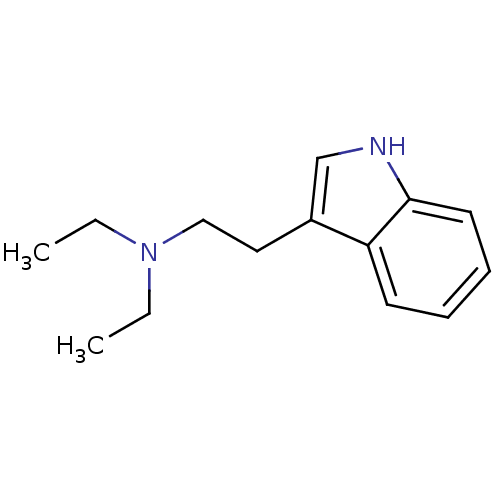 (CHEMBL142936 | Diethyl-[2-(1H-indol-3-yl)-ethyl]-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094675 (3-(2-Diethylamino-ethyl)-1H-indol-4-ol | CHEMBL143...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094674 (CHEMBL142862 | [2-(4-Fluoro-5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094669 (CHEMBL337590 | [2-(6-Fluoro-5-methoxy-1H-indol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094676 (CHEMBL142936 | Diethyl-[2-(1H-indol-3-yl)-ethyl]-a...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094671 (3-(2-Dimethylamino-ethyl)-6-fluoro-1H-indol-4-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094677 (3-(2-Dimethylamino-ethyl)-7-fluoro-1H-indol-4-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094674 (CHEMBL142862 | [2-(4-Fluoro-5-methoxy-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094676 (CHEMBL142936 | Diethyl-[2-(1H-indol-3-yl)-ethyl]-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094672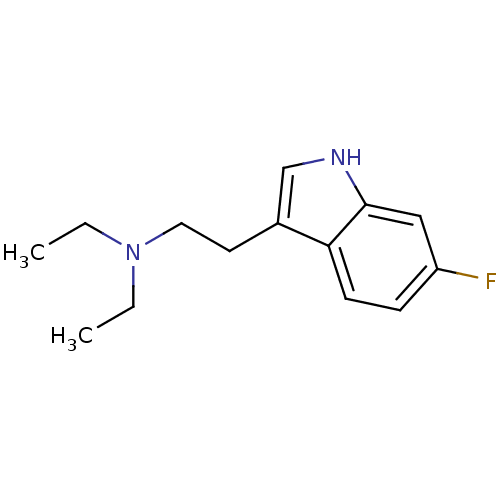 (CHEMBL444612 | Diethyl-[2-(6-fluoro-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50094672 (CHEMBL444612 | Diethyl-[2-(6-fluoro-1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [125I]-DOI-labeled rat 5-hydroxytryptamine 2C receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094672 (CHEMBL444612 | Diethyl-[2-(6-fluoro-1H-indol-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the binding constant at [3H]-8-OH-DPAT-labeled human 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM81945 (CAS_39484 | CHEMBL105891 | CHEMBL108926 | CHEMBL27...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of [3H]5-HT uptake at human SERT C109A mutant transfected in HEK cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50476261 (4-(1-Methylpyridinium)Phenylmethanethiosulphonate ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of [3H]5-HT uptake at human SERT C109A mutant transfected in HEK cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094677 (3-(2-Dimethylamino-ethyl)-7-fluoro-1H-indol-4-ol |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.92E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094676 (CHEMBL142936 | Diethyl-[2-(1H-indol-3-yl)-ethyl]-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094676 (CHEMBL142936 | Diethyl-[2-(1H-indol-3-yl)-ethyl]-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094672 (CHEMBL444612 | Diethyl-[2-(6-fluoro-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094673 (CHEMBL342986 | Diethyl-[2-(5-methoxy-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094671 (3-(2-Dimethylamino-ethyl)-6-fluoro-1H-indol-4-ol |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094669 (CHEMBL337590 | [2-(6-Fluoro-5-methoxy-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094674 (CHEMBL142862 | [2-(4-Fluoro-5-methoxy-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094672 (CHEMBL444612 | Diethyl-[2-(6-fluoro-1H-indol-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5.82 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50094675 (3-(2-Diethylamino-ethyl)-1H-indol-4-ol | CHEMBL143...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Evaluated for the effective concentration at [125I]-DOI-labeled rat 5-hydroxytryptamine 2A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50476261 (4-(1-Methylpyridinium)Phenylmethanethiosulphonate ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+13 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Decrease in [3H]5-HT uptake at human SERT W103C mutant transfected in HEK293 cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50476262 (CHEMBL224476) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Decrease in [3H]5-HT uptake at human SERT G498C mutant transfected in HEK293 cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50476262 (CHEMBL224476) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+12 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Decrease in [3H]5-HT uptake at human SERT W103C mutant transfected in HEK293 cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50476261 (4-(1-Methylpyridinium)Phenylmethanethiosulphonate ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Decrease in [3H]5-HT uptake at human SERT G498C mutant transfected in HEK293 cells | Bioorg Med Chem 15: 305-11 (2007) Article DOI: 10.1016/j.bmc.2006.09.058 BindingDB Entry DOI: 10.7270/Q2DV1NNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094670 (1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094673 (CHEMBL342986 | Diethyl-[2-(5-methoxy-1H-indol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50094674 (CHEMBL142862 | [2-(4-Fluoro-5-methoxy-1H-indol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description EC50 for inhibition of 50 microM forskolin-stimulated cAMP accumulation against 5-hydroxytryptamine 1A receptor | J Med Chem 43: 4701-10 (2001) BindingDB Entry DOI: 10.7270/Q2HT2NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||