 Found 1239 hits with Last Name = 'batt' and Initial = 'f'
Found 1239 hits with Last Name = 'batt' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85488
(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573367
(CHEMBL4865434)Show SMILES CCCN1CCC[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
Neuromedin-B receptor
(RAT) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50026917
(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50585129
(CHEMBL5077645)Show SMILES [H][C@@]12CCC[C@@](CCN1C\C=C\c1ccccc1)([C@@H]2O)c1cccc(O)c1 |r,THB:9:8:4.3.2:18| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
Bombesin
(Frog) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573366
(CHEMBL4861573)Show SMILES Cl.CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2CCCN2)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.431 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573380

(CHEMBL4873313)Show SMILES CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2C[C@H](CN2)OCCCCOc2ccc3CCC(=O)Nc3c2)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573381

(CHEMBL4864320)Show SMILES CCCN1C[C@@H](C[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC)OCCCCOc1ccc2CCC(=O)Nc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573383

(CHEMBL4850300)Show SMILES CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2C[C@H](CN2)OCCCCNC(=O)c2cc3ccccc3o2)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573385

(CHEMBL4846561)Show SMILES CCN1C[C@@H](C[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC)OCCCCNC(=O)c1cc2ccccc2o1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004769
(CHEMBL2312537)Show SMILES C(COc1ccccc1)NCC1COCC(O1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H27NO3/c1-4-10-21(11-5-1)25(22-12-6-2-7-13-22)20-27-19-24(29-25)18-26-16-17-28-23-14-8-3-9-15-23/h1-15,24,26H,16-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50585128

(CHEMBL5080654)Show SMILES [H][C@@]12CCC[C@@](CCN1CCc1ccccc1)(\C2=C\C(=O)OCC)c1cccc(O)c1 |r,THB:9:8:4.3.2:17| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.633 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004770
(CHEMBL2312227)Show SMILES COc1ccccc1OCCNCC1COCC(O1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H29NO4/c1-28-24-14-8-9-15-25(24)30-17-16-27-18-23-19-29-20-26(31-23,21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,27H,16-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50573367

(CHEMBL4865434)Show SMILES CCCN1CCC[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM85480
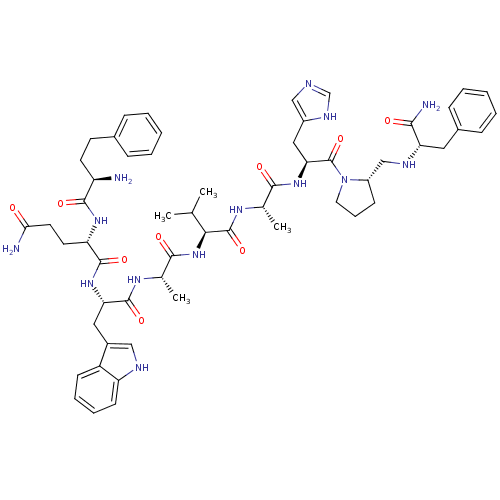
((3-Ph-Pr6)His7,DAla11,DPro13,Psi13-14,Phe14-Bn(6-1...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1CN[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H76N14O9/c1-33(2)49(56(79)66-34(3)51(74)69-47(28-39-30-61-32-64-39)57(80)71-25-13-18-40(71)31-63-45(50(60)73)26-37-16-9-6-10-17-37)70-52(75)35(4)65-55(78)46(27-38-29-62-43-20-12-11-19-41(38)43)68-54(77)44(23-24-48(59)72)67-53(76)42(58)22-21-36-14-7-5-8-15-36/h5-12,14-17,19-20,29-30,32-35,40,42,44-47,49,62-63H,13,18,21-28,31,58H2,1-4H3,(H2,59,72)(H2,60,73)(H,61,64)(H,65,78)(H,66,79)(H,67,76)(H,68,77)(H,69,74)(H,70,75)/t34-,35-,40-,42+,44-,45-,46-,47-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM85500
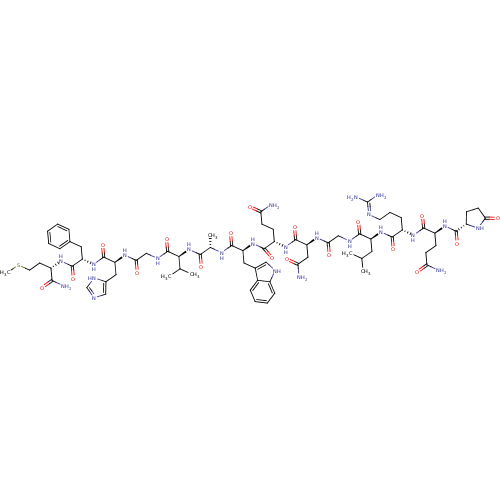
(Bombesin,Phe13)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:85.96,77.85,8.16,4.4,33.35,56.65,19.27,wD:96.105,42.56,65.73,105.108,37.39,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;28.74,-16.5,;30.07,-17.27,;31.4,-16.5,;31.4,-14.96,;30.07,-14.19,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.62,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.5,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C74H108N24O18S/c1-37(2)27-50(95-65(108)46(17-12-25-82-74(79)80)92-67(110)48(18-21-55(75)99)93-66(109)47-20-23-58(102)88-47)64(107)84-34-59(103)90-54(31-57(77)101)72(115)94-49(19-22-56(76)100)68(111)97-52(29-41-32-83-44-16-11-10-15-43(41)44)69(112)87-39(5)63(106)98-61(38(3)4)73(116)85-35-60(104)89-53(30-42-33-81-36-86-42)71(114)96-51(28-40-13-8-7-9-14-40)70(113)91-45(62(78)105)24-26-117-6/h7-11,13-16,32-33,36-39,45-54,61,83H,12,17-31,34-35H2,1-6H3,(H2,75,99)(H2,76,100)(H2,77,101)(H2,78,105)(H,81,86)(H,84,107)(H,85,116)(H,87,112)(H,88,102)(H,89,104)(H,90,103)(H,91,113)(H,92,110)(H,93,109)(H,94,115)(H,95,108)(H,96,114)(H,97,111)(H,98,106)(H4,79,80,82)/t39-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,61-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50079412
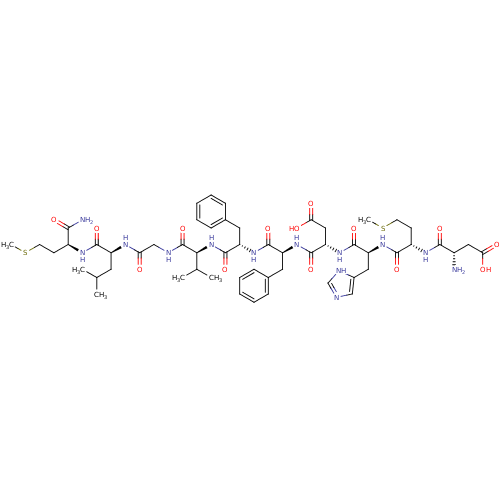
((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C55H79N13O14S2/c1-30(2)21-38(50(77)62-36(47(57)74)17-19-83-5)61-43(69)28-59-55(82)46(31(3)4)68-54(81)40(23-33-15-11-8-12-16-33)65-51(78)39(22-32-13-9-7-10-14-32)64-53(80)42(26-45(72)73)67-52(79)41(24-34-27-58-29-60-34)66-49(76)37(18-20-84-6)63-48(75)35(56)25-44(70)71/h7-16,27,29-31,35-42,46H,17-26,28,56H2,1-6H3,(H2,57,74)(H,58,60)(H,59,82)(H,61,69)(H,62,77)(H,63,75)(H,64,80)(H,65,78)(H,66,76)(H,67,79)(H,68,81)(H,70,71)(H,72,73)/t35-,36-,37-,38-,39-,40-,41-,42-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA
Curated by ChEMBL
| Assay Description
Displacement of [125I][MePhe7]NKB from human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis |
J Med Chem 58: 3060-82 (2015)
Article DOI: 10.1021/jm5017413
BindingDB Entry DOI: 10.7270/Q2ZP47TC |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573382

(CHEMBL4860478)Show SMILES CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2C[C@H](CN2)OCCCCNC(=O)c2cc3ccccc3[nH]2)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.797 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573388

(CHEMBL4847432)Show SMILES CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2C[C@H](CN2)OCCCCO)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50585107

(CHEMBL5085974)Show SMILES CN(C)C(=O)C(CCN1CCN(CC1)c1cccc(Cl)c1Cl)(c1ccccc1)c1ccccc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50585112
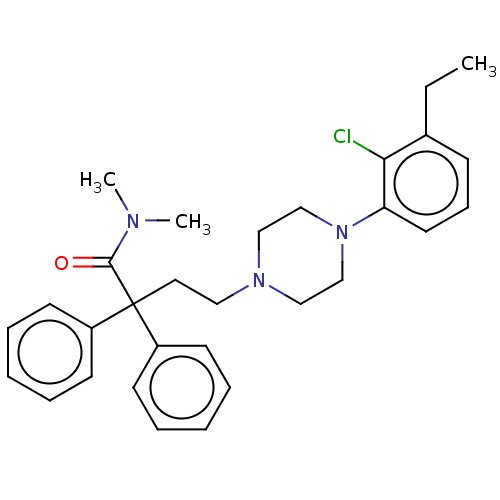
(CHEMBL5088070)Show SMILES CCc1cccc(N2CCN(CCC(C(=O)N(C)C)(c3ccccc3)c3ccccc3)CC2)c1Cl | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cell membrane incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50515232
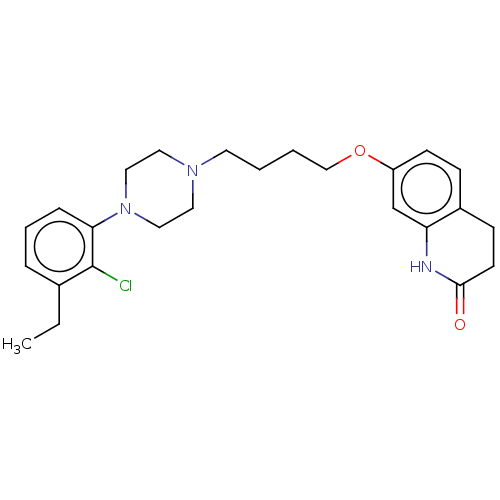
(CHEMBL4471817)Show SMILES CCc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C25H32ClN3O2/c1-2-19-6-5-7-23(25(19)26)29-15-13-28(14-16-29)12-3-4-17-31-21-10-8-20-9-11-24(30)27-22(20)18-21/h5-8,10,18H,2-4,9,11-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.844 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-(+)-7-OH-DPAT from recombinant human D2L receptor expressed in HEK293 cell membranes measured after 90 mins by microbeta sci... |
J Med Chem 62: 6287-6314 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00702
BindingDB Entry DOI: 10.7270/Q2X63R9B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50573385

(CHEMBL4846561)Show SMILES CCN1C[C@@H](C[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC)OCCCCNC(=O)c1cc2ccccc2o1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.947 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Bombesin
(Frog) | BDBM85500

(Bombesin,Phe13)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:85.96,77.85,8.16,4.4,33.35,56.65,19.27,wD:96.105,42.56,65.73,105.108,37.39,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;28.74,-16.5,;30.07,-17.27,;31.4,-16.5,;31.4,-14.96,;30.07,-14.19,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.62,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.5,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C74H108N24O18S/c1-37(2)27-50(95-65(108)46(17-12-25-82-74(79)80)92-67(110)48(18-21-55(75)99)93-66(109)47-20-23-58(102)88-47)64(107)84-34-59(103)90-54(31-57(77)101)72(115)94-49(19-22-56(76)100)68(111)97-52(29-41-32-83-44-16-11-10-15-43(41)44)69(112)87-39(5)63(106)98-61(38(3)4)73(116)85-35-60(104)89-53(30-42-33-81-36-86-42)71(114)96-51(28-40-13-8-7-9-14-40)70(113)91-45(62(78)105)24-26-117-6/h7-11,13-16,32-33,36-39,45-54,61,83H,12,17-31,34-35H2,1-6H3,(H2,75,99)(H2,76,100)(H2,77,101)(H2,78,105)(H,81,86)(H,84,107)(H,85,116)(H,87,112)(H,88,102)(H,89,104)(H,90,103)(H,91,113)(H,92,110)(H,93,109)(H,94,115)(H,95,108)(H,96,114)(H,97,111)(H,98,106)(H4,79,80,82)/t39-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,61-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM85488

(DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14))Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34-,40+,42-,43-,44-,45-,46-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573387

(CHEMBL4856983)Show SMILES CCc1cc(Cl)c(OC)c(C(=O)NC[C@@H]2C[C@H](CN2)OCCCCOCc2ccccc2)c1O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50004770

(CHEMBL2312227)Show SMILES COc1ccccc1OCCNCC1COCC(O1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H29NO4/c1-28-24-14-8-9-15-25(24)30-17-16-27-18-23-19-29-20-26(31-23,21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,27H,16-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]Prazosin from human recombinant alpha 1D adrenergic receptor expressed in CHO cells incubated for 30 mins by radioligand competit... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50573384
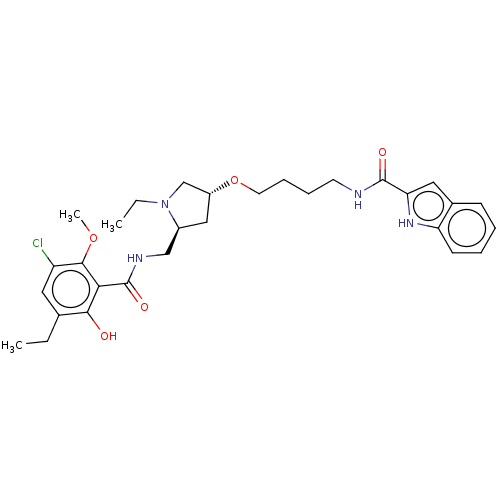
(CHEMBL4853568)Show SMILES CCN1C[C@@H](C[C@H]1CNC(=O)c1c(O)c(CC)cc(Cl)c1OC)OCCCCNC(=O)c1cc2ccccc2[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01353
BindingDB Entry DOI: 10.7270/Q2BV7MF8 |
More data for this
Ligand-Target Pair | |
Bombesin
(Frog) | BDBM85498
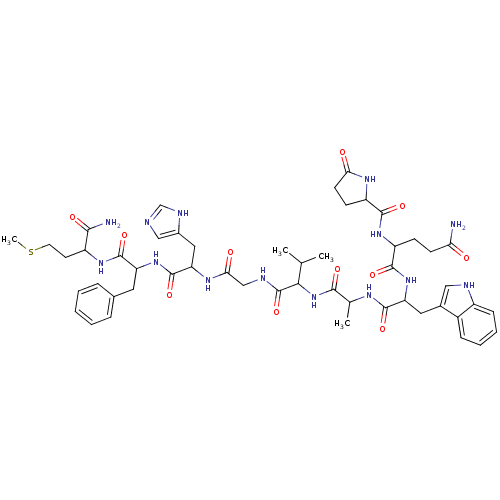
(CAS_5486808 | Litorin | NSC_5486808)Show SMILES CSCCC(NC(=O)C(Cc1ccccc1)NC(=O)C(Cc1cnc[nH]1)NC(=O)CNC(=O)C(NC(=O)C(C)NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(CCC(N)=O)NC(=O)C1CCC(=O)N1)C(C)C)C(N)=O Show InChI InChI=1S/C51H68N14O11S/c1-27(2)43(51(76)56-25-42(68)60-39(22-31-24-54-26-57-31)50(75)63-37(20-29-10-6-5-7-11-29)49(74)61-34(44(53)69)18-19-77-4)65-45(70)28(3)58-48(73)38(21-30-23-55-33-13-9-8-12-32(30)33)64-47(72)36(14-16-40(52)66)62-46(71)35-15-17-41(67)59-35/h5-13,23-24,26-28,34-39,43,55H,14-22,25H2,1-4H3,(H2,52,66)(H2,53,69)(H,54,57)(H,56,76)(H,58,73)(H,59,67)(H,60,68)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,70) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85481
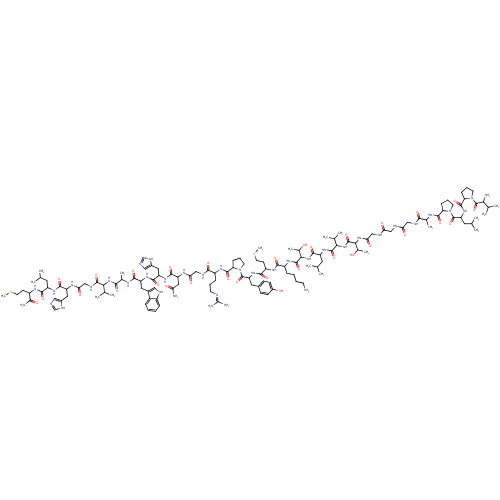
(CAS_93755-85-2 | GRP)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CNC(=O)C(NC(=O)C(C)NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(CC(N)=O)NC(=O)CNC(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(Cc1ccc(O)cc1)NC(=O)C(CCSC)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(NC(=O)CNC(=O)CNC(=O)CNC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(C)C)NC(=O)C1CCCN1C(=O)C(N)C(C)C)C(C)O)C(C)C)C(C)O)C(C)C)C(N)=O |(76.66,-17.73,;77.14,-16.27,;76.11,-15.12,;76.59,-13.66,;75.56,-12.51,;76.04,-11.05,;77.54,-10.73,;78.57,-11.88,;78.02,-9.27,;79.53,-8.95,;80.56,-10.1,;82.07,-9.78,;80.08,-11.56,;76.99,-8.12,;77.47,-6.66,;78.98,-6.34,;76.44,-5.51,;76.92,-4.05,;78.43,-3.73,;79.06,-2.33,;80.59,-2.49,;80.91,-4,;79.57,-4.76,;74.94,-5.83,;73.91,-4.68,;74.39,-3.22,;72.4,-5,;71.37,-3.86,;69.87,-4.17,;69.39,-5.64,;68.84,-3.03,;67.33,-3.34,;66.3,-2.2,;66.78,-.73,;64.8,-2.52,;64.32,-3.98,;63.77,-1.37,;62.26,-1.69,;61.78,-3.15,;61.23,-.54,;59.73,-.86,;59.25,-2.32,;60.15,-3.56,;59.25,-4.8,;57.79,-4.33,;56.46,-5.1,;55.12,-4.33,;55.12,-2.79,;56.46,-2.02,;57.79,-2.79,;61.71,.92,;60.68,2.07,;59.18,1.75,;61.16,3.53,;62.67,3.85,;63.15,5.31,;64.61,5.79,;64.61,7.33,;63.15,7.81,;62.24,6.56,;60.13,4.68,;58.62,4.36,;58.15,2.9,;57.6,5.51,;58.07,6.97,;57.05,8.12,;57.52,9.58,;55.54,7.8,;56.09,5.19,;55.61,3.73,;56.64,2.58,;54.1,3.41,;53.63,1.94,;52.12,1.63,;51.09,2.77,;51.64,.16,;52.67,-.98,;52.19,-2.45,;53.22,-3.59,;52.74,-5.06,;53.77,-6.2,;53.29,-7.67,;55.28,-5.89,;50.13,-.15,;49.66,-1.62,;50.68,-2.76,;48.15,-1.94,;47.52,-3.34,;45.99,-3.18,;45.67,-1.67,;47.01,-.9,;47.17,.63,;48.58,1.25,;45.92,1.54,;46.09,3.07,;44.84,3.97,;43.44,3.35,;42.19,4.26,;42.36,5.79,;41.11,6.7,;43.76,6.41,;45.01,5.51,;44.52,.91,;43.41,-.16,;43.78,-1.65,;41.93,.27,;41.56,1.77,;40.08,2.19,;39.71,3.69,;38.23,4.12,;40.82,-.8,;39.34,-.37,;38.97,1.13,;38.23,-1.44,;38.6,-2.93,;37.49,-4,;37.86,-5.5,;36.75,-6.56,;37.12,-8.06,;36.75,-1.01,;35.64,-2.08,;36.01,-3.57,;34.16,-1.65,;33.05,-2.72,;31.57,-2.29,;31.2,-.8,;30.46,-3.36,;30.83,-4.86,;29.72,-5.92,;30.09,-7.42,;28.24,-5.5,;28.98,-2.93,;28.61,-1.44,;29.72,-.37,;27.13,-1.01,;26.76,.48,;25.28,.91,;24.17,-.16,;24.91,2.41,;23.43,2.83,;23.06,4.33,;24.17,5.4,;21.58,4.75,;21.21,6.25,;19.73,6.68,;18.62,5.61,;19.36,8.17,;17.88,8.6,;17.51,10.09,;18.62,11.16,;16.03,10.52,;15.66,12.02,;14.18,12.44,;13.07,11.37,;13.81,13.94,;14.92,15.01,;12.33,14.36,;11.96,15.86,;13.07,16.93,;10.48,16.29,;9.96,17.73,;8.42,17.68,;7.99,16.2,;9.27,15.34,;9.32,13.8,;10.68,13.08,;8.01,12.99,;6.65,13.71,;5.35,12.9,;3.99,13.62,;5.4,11.36,;8.06,11.45,;9.42,10.72,;10.73,11.54,;9.47,9.18,;10.75,8.32,;10.32,6.84,;8.78,6.79,;8.26,8.24,;6.78,8.66,;6.41,10.16,;5.67,7.6,;6.04,6.1,;4.19,8.02,;3.08,6.96,;3.82,9.52,;26.02,3.47,;27.5,3.05,;25.65,4.97,;26.02,-2.08,;24.54,-1.65,;26.39,-3.57,;33.79,-.16,;34.9,.91,;32.31,.27,;69.32,-1.56,;70.82,-1.25,;68.29,-.42,;74.05,-12.83,;73.57,-14.3,;73.02,-11.69,)| Show InChI InChI=1S/C130H204N38O31S2/c1-65(2)47-86(115(185)152-82(108(134)178)38-45-200-17)156-116(186)89(52-77-56-137-63-146-77)150-101(176)62-145-123(193)104(69(9)10)163-110(180)72(14)148-114(184)88(51-76-55-140-81-28-20-19-27-80(76)81)157-117(187)90(53-78-57-138-64-147-78)158-118(188)91(54-97(132)172)151-100(175)61-144-111(181)83(30-23-41-139-130(135)136)154-121(191)95-32-25-43-167(95)128(198)93(50-75-34-36-79(171)37-35-75)160-113(183)85(39-46-201-18)153-112(182)84(29-21-22-40-131)155-125(195)107(74(16)170)165-119(189)87(48-66(3)4)159-124(194)105(70(11)12)164-126(196)106(73(15)169)162-102(177)60-142-98(173)58-141-99(174)59-143-109(179)71(13)149-120(190)94-31-24-42-166(94)127(197)92(49-67(5)6)161-122(192)96-33-26-44-168(96)129(199)103(133)68(7)8/h19-20,27-28,34-37,55-57,63-74,82-96,103-107,140,169-171H,21-26,29-33,38-54,58-62,131,133H2,1-18H3,(H2,132,172)(H2,134,178)(H,137,146)(H,138,147)(H,141,174)(H,142,173)(H,143,179)(H,144,181)(H,145,193)(H,148,184)(H,149,190)(H,150,176)(H,151,175)(H,152,185)(H,153,182)(H,154,191)(H,155,195)(H,156,186)(H,157,187)(H,158,188)(H,159,194)(H,160,183)(H,161,192)(H,162,177)(H,163,180)(H,164,196)(H,165,189)(H4,135,136,139) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85484
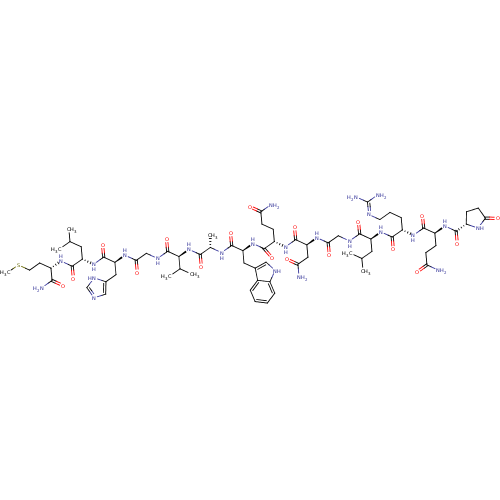
(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50116766

((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...)Show InChI InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-(+)-7-OH-DPAT from recombinant human D3 receptor expressed in HEK293 cell membranes measured after 90 mins by microbeta scin... |
J Med Chem 62: 6287-6314 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00702
BindingDB Entry DOI: 10.7270/Q2X63R9B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]Prazosin from human recombinant alpha 1D adrenergic receptor expressed in CHO cells incubated for 30 mins by radioligand competit... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85484

(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85484

(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50004772
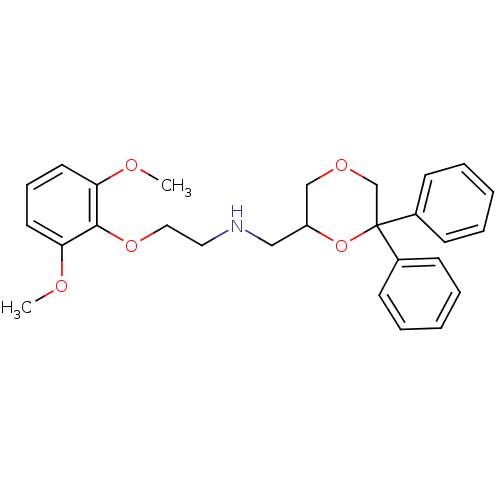
(CHEMBL2312225)Show SMILES COc1cccc(OC)c1OCCNCC1COCC(O1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H31NO5/c1-29-24-14-9-15-25(30-2)26(24)32-17-16-28-18-23-19-31-20-27(33-23,21-10-5-3-6-11-21)22-12-7-4-8-13-22/h3-15,23,28H,16-20H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human recombinant 5-HT1A receptor expressed in human HeLa cells incubated for 30 mins by radioligand competition ... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylspiperone from human dopamine D4 receptor expressed in HEK293 cell membranes incubated for 60 mins by microbeta scintill... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00611
BindingDB Entry DOI: 10.7270/Q2N301V5 |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM85481

(CAS_93755-85-2 | GRP)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CNC(=O)C(NC(=O)C(C)NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(CC(N)=O)NC(=O)CNC(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(Cc1ccc(O)cc1)NC(=O)C(CCSC)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(NC(=O)CNC(=O)CNC(=O)CNC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(C)C)NC(=O)C1CCCN1C(=O)C(N)C(C)C)C(C)O)C(C)C)C(C)O)C(C)C)C(N)=O |(76.66,-17.73,;77.14,-16.27,;76.11,-15.12,;76.59,-13.66,;75.56,-12.51,;76.04,-11.05,;77.54,-10.73,;78.57,-11.88,;78.02,-9.27,;79.53,-8.95,;80.56,-10.1,;82.07,-9.78,;80.08,-11.56,;76.99,-8.12,;77.47,-6.66,;78.98,-6.34,;76.44,-5.51,;76.92,-4.05,;78.43,-3.73,;79.06,-2.33,;80.59,-2.49,;80.91,-4,;79.57,-4.76,;74.94,-5.83,;73.91,-4.68,;74.39,-3.22,;72.4,-5,;71.37,-3.86,;69.87,-4.17,;69.39,-5.64,;68.84,-3.03,;67.33,-3.34,;66.3,-2.2,;66.78,-.73,;64.8,-2.52,;64.32,-3.98,;63.77,-1.37,;62.26,-1.69,;61.78,-3.15,;61.23,-.54,;59.73,-.86,;59.25,-2.32,;60.15,-3.56,;59.25,-4.8,;57.79,-4.33,;56.46,-5.1,;55.12,-4.33,;55.12,-2.79,;56.46,-2.02,;57.79,-2.79,;61.71,.92,;60.68,2.07,;59.18,1.75,;61.16,3.53,;62.67,3.85,;63.15,5.31,;64.61,5.79,;64.61,7.33,;63.15,7.81,;62.24,6.56,;60.13,4.68,;58.62,4.36,;58.15,2.9,;57.6,5.51,;58.07,6.97,;57.05,8.12,;57.52,9.58,;55.54,7.8,;56.09,5.19,;55.61,3.73,;56.64,2.58,;54.1,3.41,;53.63,1.94,;52.12,1.63,;51.09,2.77,;51.64,.16,;52.67,-.98,;52.19,-2.45,;53.22,-3.59,;52.74,-5.06,;53.77,-6.2,;53.29,-7.67,;55.28,-5.89,;50.13,-.15,;49.66,-1.62,;50.68,-2.76,;48.15,-1.94,;47.52,-3.34,;45.99,-3.18,;45.67,-1.67,;47.01,-.9,;47.17,.63,;48.58,1.25,;45.92,1.54,;46.09,3.07,;44.84,3.97,;43.44,3.35,;42.19,4.26,;42.36,5.79,;41.11,6.7,;43.76,6.41,;45.01,5.51,;44.52,.91,;43.41,-.16,;43.78,-1.65,;41.93,.27,;41.56,1.77,;40.08,2.19,;39.71,3.69,;38.23,4.12,;40.82,-.8,;39.34,-.37,;38.97,1.13,;38.23,-1.44,;38.6,-2.93,;37.49,-4,;37.86,-5.5,;36.75,-6.56,;37.12,-8.06,;36.75,-1.01,;35.64,-2.08,;36.01,-3.57,;34.16,-1.65,;33.05,-2.72,;31.57,-2.29,;31.2,-.8,;30.46,-3.36,;30.83,-4.86,;29.72,-5.92,;30.09,-7.42,;28.24,-5.5,;28.98,-2.93,;28.61,-1.44,;29.72,-.37,;27.13,-1.01,;26.76,.48,;25.28,.91,;24.17,-.16,;24.91,2.41,;23.43,2.83,;23.06,4.33,;24.17,5.4,;21.58,4.75,;21.21,6.25,;19.73,6.68,;18.62,5.61,;19.36,8.17,;17.88,8.6,;17.51,10.09,;18.62,11.16,;16.03,10.52,;15.66,12.02,;14.18,12.44,;13.07,11.37,;13.81,13.94,;14.92,15.01,;12.33,14.36,;11.96,15.86,;13.07,16.93,;10.48,16.29,;9.96,17.73,;8.42,17.68,;7.99,16.2,;9.27,15.34,;9.32,13.8,;10.68,13.08,;8.01,12.99,;6.65,13.71,;5.35,12.9,;3.99,13.62,;5.4,11.36,;8.06,11.45,;9.42,10.72,;10.73,11.54,;9.47,9.18,;10.75,8.32,;10.32,6.84,;8.78,6.79,;8.26,8.24,;6.78,8.66,;6.41,10.16,;5.67,7.6,;6.04,6.1,;4.19,8.02,;3.08,6.96,;3.82,9.52,;26.02,3.47,;27.5,3.05,;25.65,4.97,;26.02,-2.08,;24.54,-1.65,;26.39,-3.57,;33.79,-.16,;34.9,.91,;32.31,.27,;69.32,-1.56,;70.82,-1.25,;68.29,-.42,;74.05,-12.83,;73.57,-14.3,;73.02,-11.69,)| Show InChI InChI=1S/C130H204N38O31S2/c1-65(2)47-86(115(185)152-82(108(134)178)38-45-200-17)156-116(186)89(52-77-56-137-63-146-77)150-101(176)62-145-123(193)104(69(9)10)163-110(180)72(14)148-114(184)88(51-76-55-140-81-28-20-19-27-80(76)81)157-117(187)90(53-78-57-138-64-147-78)158-118(188)91(54-97(132)172)151-100(175)61-144-111(181)83(30-23-41-139-130(135)136)154-121(191)95-32-25-43-167(95)128(198)93(50-75-34-36-79(171)37-35-75)160-113(183)85(39-46-201-18)153-112(182)84(29-21-22-40-131)155-125(195)107(74(16)170)165-119(189)87(48-66(3)4)159-124(194)105(70(11)12)164-126(196)106(73(15)169)162-102(177)60-142-98(173)58-141-99(174)59-143-109(179)71(13)149-120(190)94-31-24-42-166(94)127(197)92(49-67(5)6)161-122(192)96-33-26-44-168(96)129(199)103(133)68(7)8/h19-20,27-28,34-37,55-57,63-74,82-96,103-107,140,169-171H,21-26,29-33,38-54,58-62,131,133H2,1-18H3,(H2,132,172)(H2,134,178)(H,137,146)(H,138,147)(H,141,174)(H,142,173)(H,143,179)(H,144,181)(H,145,193)(H,148,184)(H,149,190)(H,150,176)(H,151,175)(H,152,185)(H,153,182)(H,154,191)(H,155,195)(H,156,186)(H,157,187)(H,158,188)(H,159,194)(H,160,183)(H,161,192)(H,162,177)(H,163,180)(H,164,196)(H,165,189)(H4,135,136,139) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
Biochemistry 38: 7307-20 (1999)
Article DOI: 10.1021/bi990204w
BindingDB Entry DOI: 10.7270/Q29022BG |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261
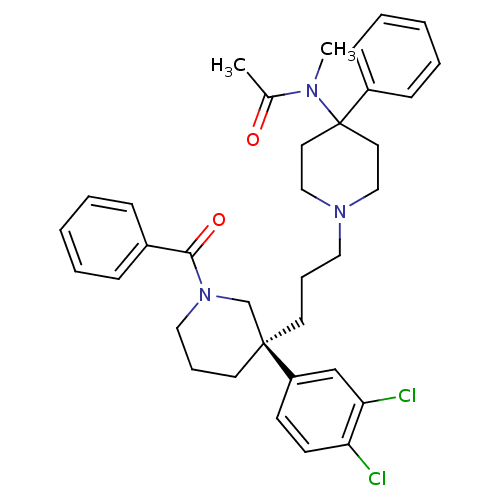
((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis |
J Med Chem 58: 3060-82 (2015)
Article DOI: 10.1021/jm5017413
BindingDB Entry DOI: 10.7270/Q2ZP47TC |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50010586
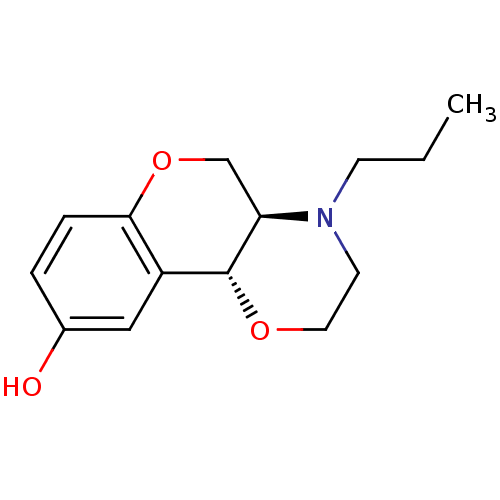
((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...)Show InChI InChI=1S/C14H19NO3/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(R)-(+)-7-OH-DPAT from human D3 receptor expressed in HEK293 cells after 90 mins by microbeta counting based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00660
BindingDB Entry DOI: 10.7270/Q2M61Q0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1D adrenergic receptor
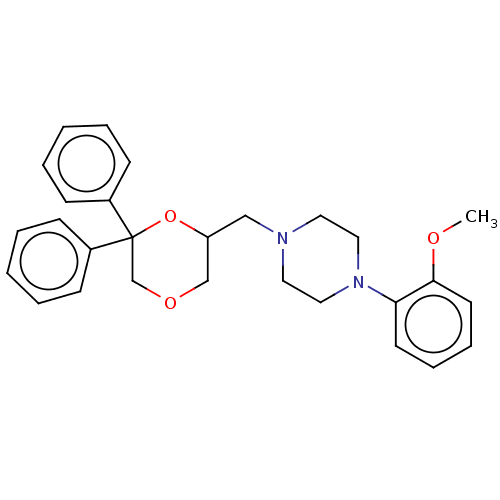
(Homo sapiens (Human)) | BDBM50523359
(CHEMBL4466677)Show SMILES COc1ccccc1N1CCN(CC2COCC(O2)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C28H32N2O3/c1-31-27-15-9-8-14-26(27)30-18-16-29(17-19-30)20-25-21-32-22-28(33-25,23-10-4-2-5-11-23)24-12-6-3-7-13-24/h2-15,25H,16-22H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]Prazosin from human recombinant alpha 1D adrenergic receptor expressed in CHO cells incubated for 30 mins by radioligand competit... |
Eur J Med Chem 168: 461-473 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.056
BindingDB Entry DOI: 10.7270/Q2WM1HT7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data