

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50112584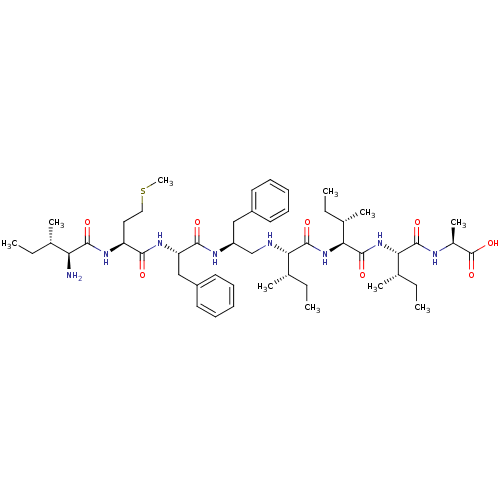 (2-(2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370072 (CHEMBL1790422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112580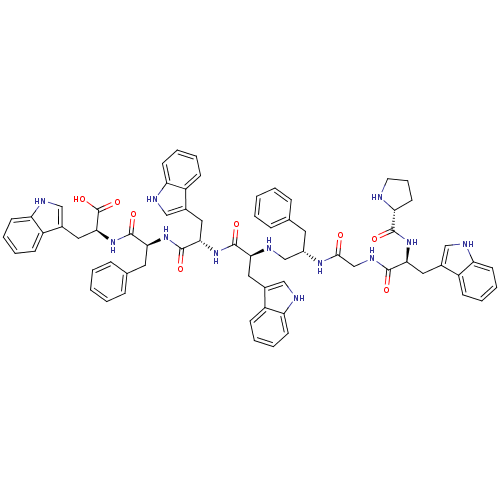 (CHEMBL268148 | PWGF(CH2NH)WWFW) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112597 (2-{2-[2-(2-{2-[2-[2-(2-Amino-3-carboxy-propionylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112581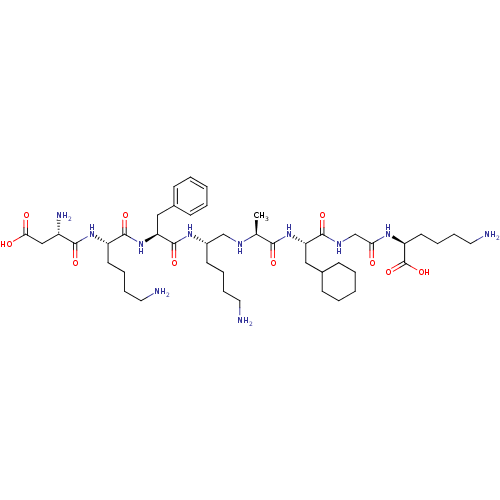 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112596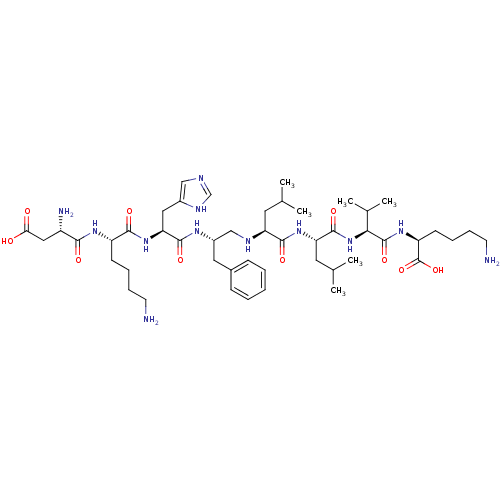 (6-Amino-2-{2-[2-(2-{2-[2-[6-amino-2-(2-amino-3-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112594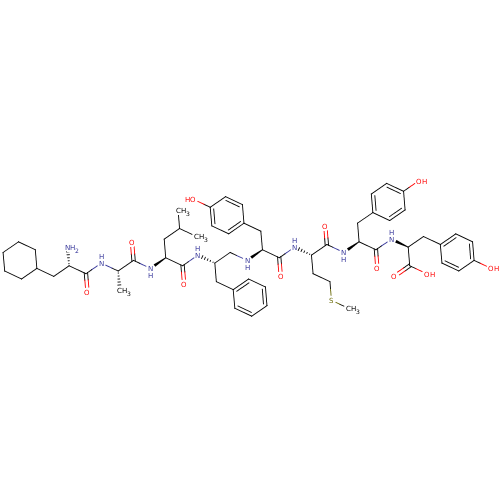 (CHEMBL266692 | ChaALF(CH2NH)YMYY) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112583 (1-{2-[2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112579 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[2-(2-amino-3-cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112591 (2-(2-{2-[2-(2-{2-[2-(2-Amino-4-methylsulfanyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112585 (2-[3-Cyclohexyl-2-(2-{2-[2-(3-(3H-imidazol-4-yl)-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112582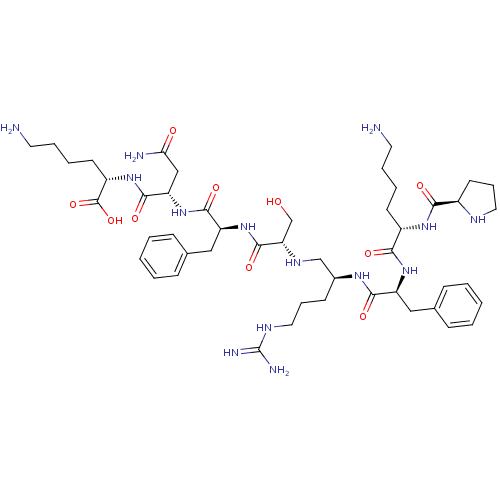 (6-Amino-2-[2-(2-{2-[2-(2-{6-amino-2-[(pyrrolidine-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370073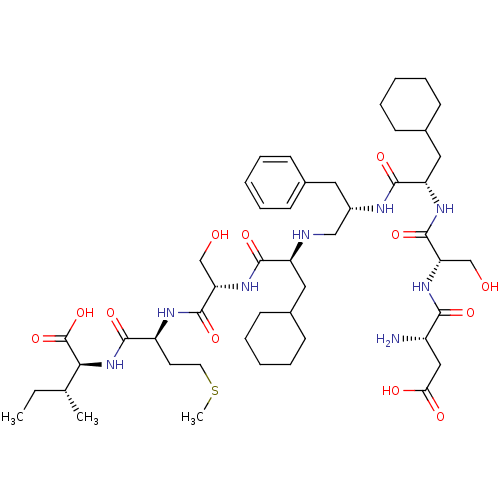 (CHEMBL1790421) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370068 (CHEMBL1790423) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370070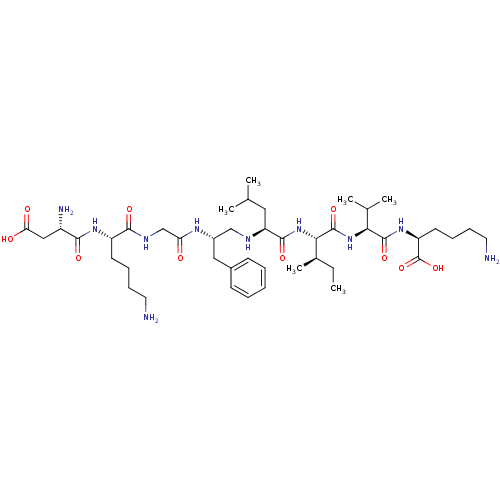 (CHEMBL1790419) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370074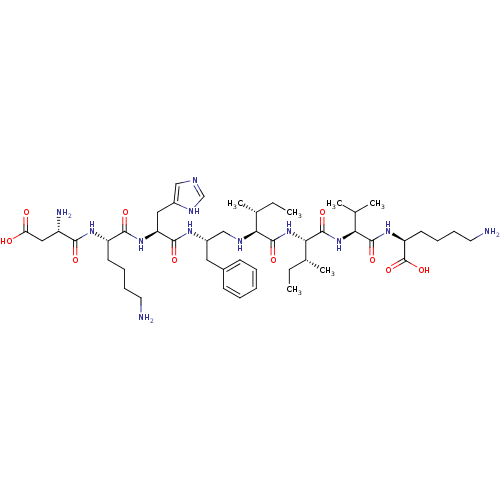 (CHEMBL1790420) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370069 (CHEMBL1790418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112592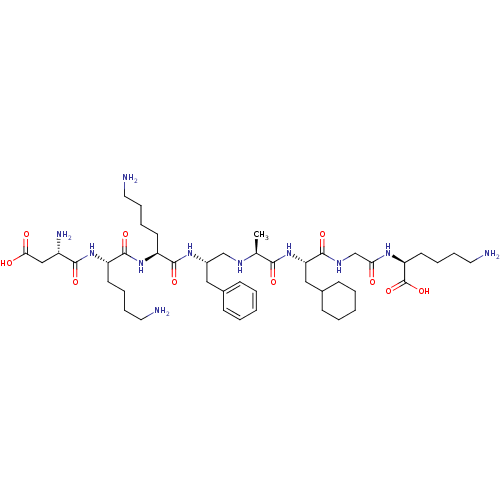 (6-Amino-2-(2-{2-[2-(2-{6-amino-2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112587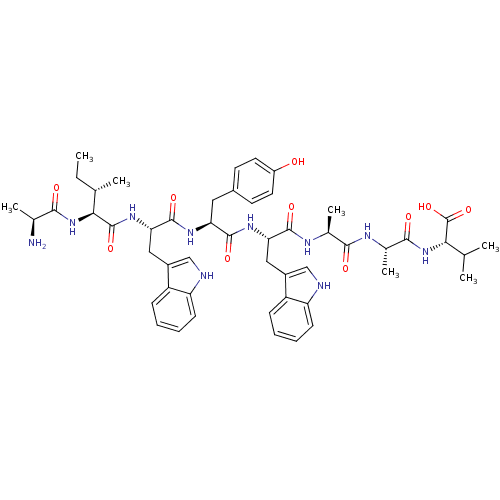 (2-(2-{2-[2-[2-[2-[2-(2-Amino-propionylamino)-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370071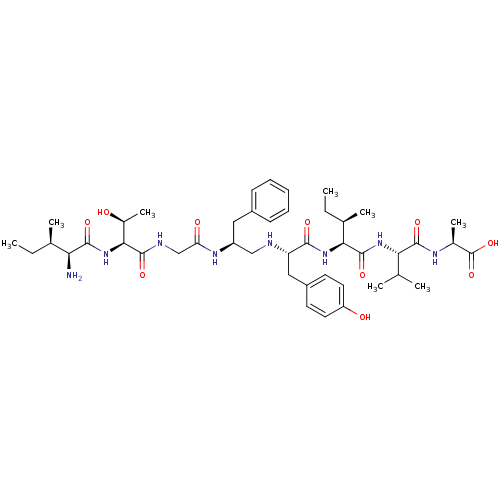 (CHEMBL1790424) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259706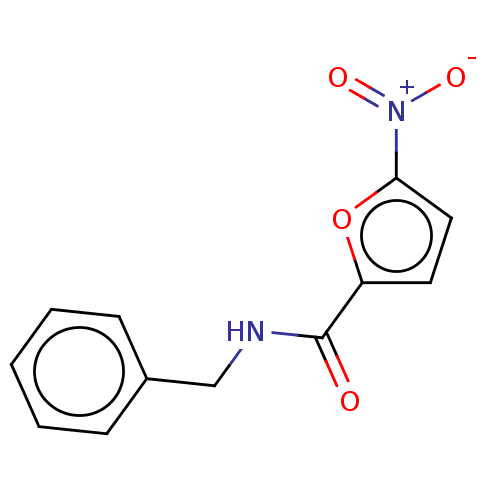 (CHEMBL2313314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259707 (CHEMBL4091464) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112588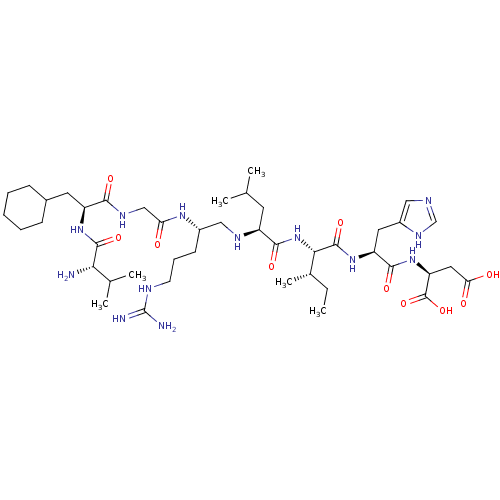 (2-[2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-butyrylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112578 (2-(2-{2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259704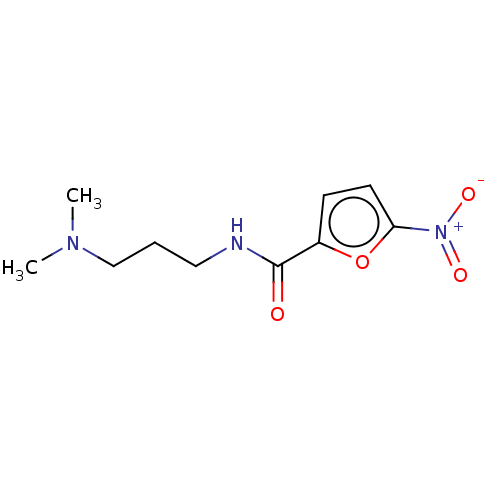 (CHEMBL4066587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259705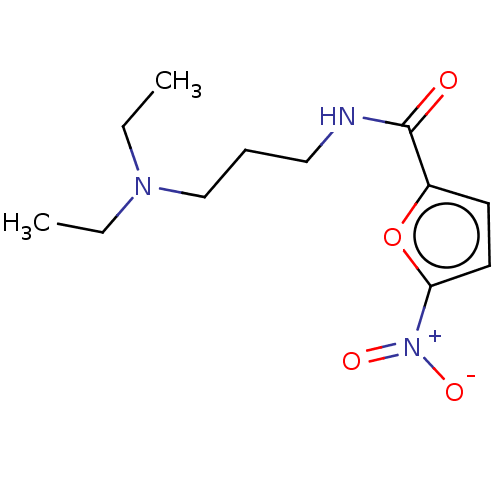 (CHEMBL4088638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259703 (CHEMBL4084206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259702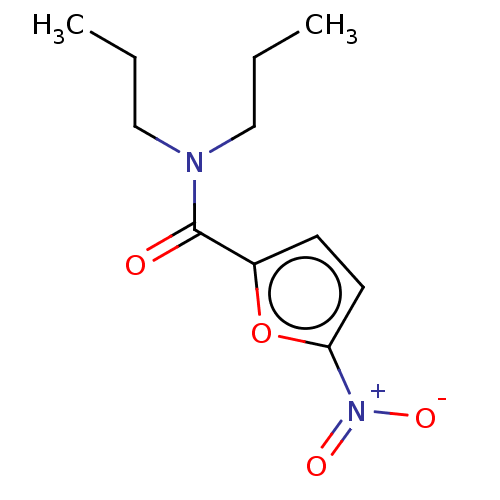 (CHEMBL4079787) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259710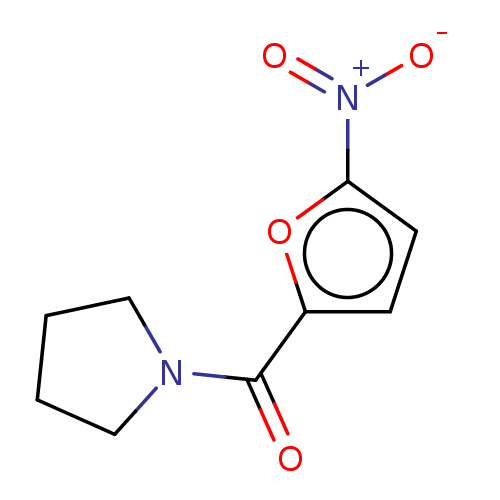 (CHEMBL4071417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259708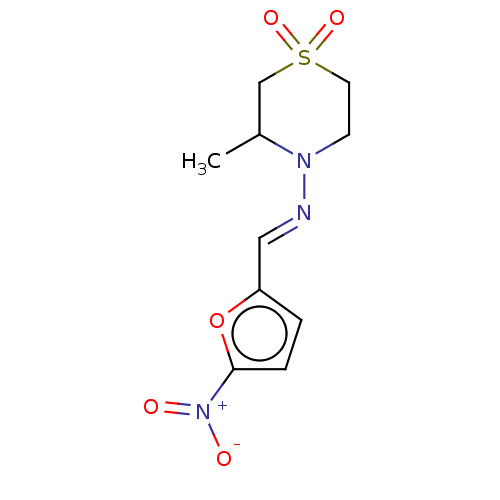 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259698 (CHEMBL4092991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259709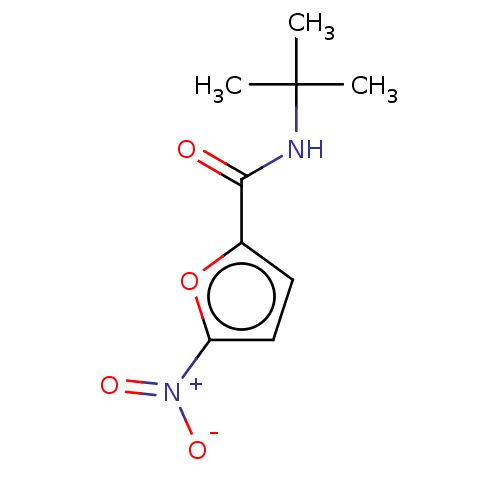 (CHEMBL4061646) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259700 (CHEMBL4064356) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259699 (CHEMBL4079946) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259711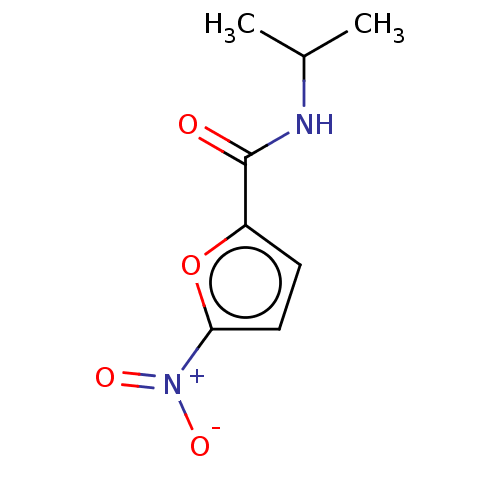 (CHEMBL4092070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259701 (CHEMBL4103302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240227 (CHEMBL4084391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240209 (CHEMBL4068526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240213 (CHEMBL4085366) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240224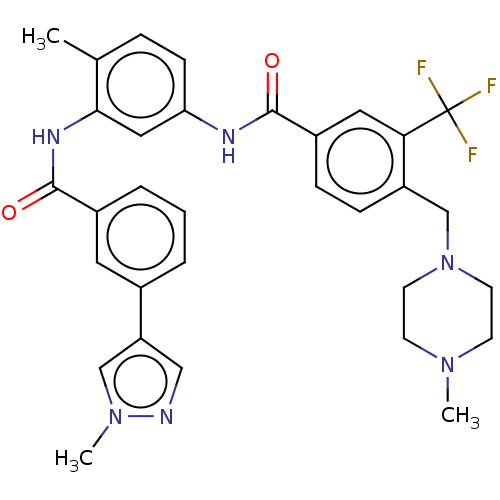 (CHEMBL4095856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240216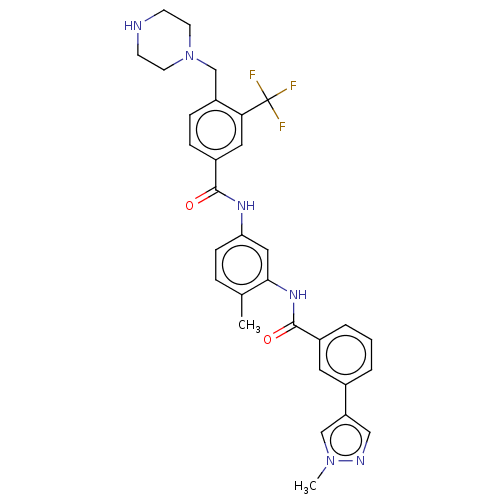 (CHEMBL4074076) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240226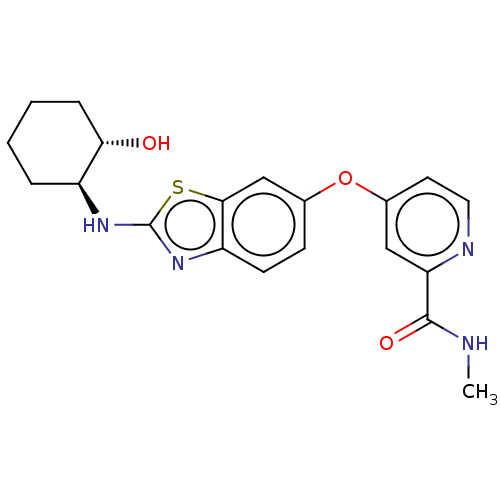 (CHEMBL4065459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Ribonucleoside diphosphate reductase was determined | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405407 (CHEMBL5285361) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405402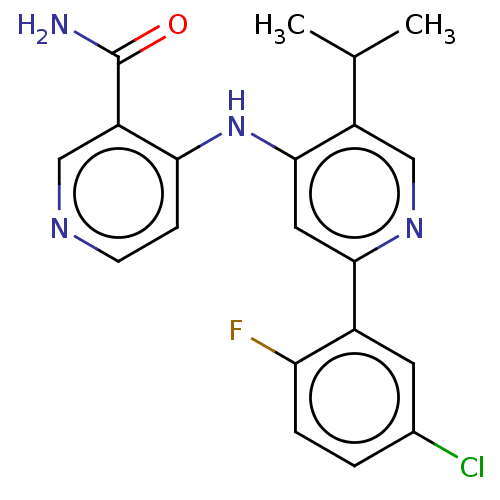 (CHEMBL5267945) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240218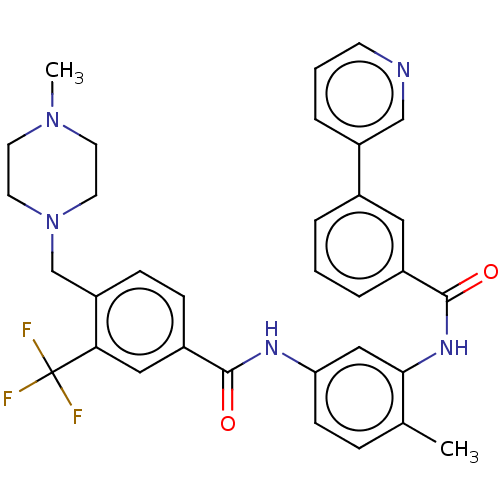 (CHEMBL4059858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405385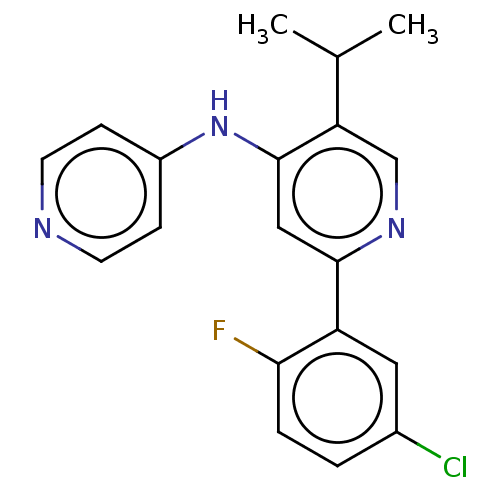 (CHEMBL5285746) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50405403 (CHEMBL5274165) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM514529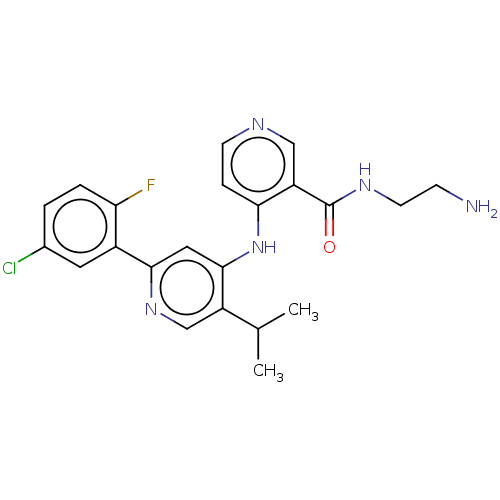 (N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli leader peptidase using substrate A | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240227 (CHEMBL4084391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50240219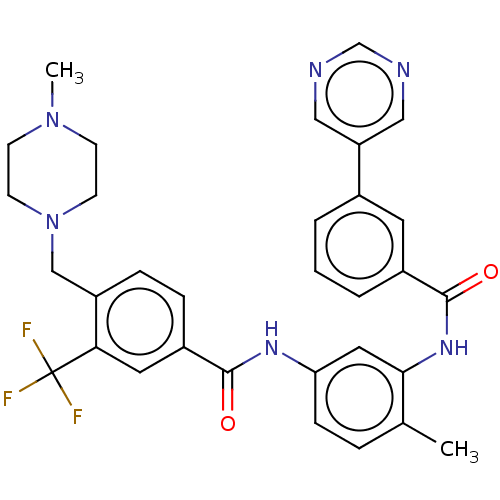 (CHEMBL4100838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd Curated by ChEMBL | Assay Description Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... | Bioorg Med Chem Lett 27: 2153-2160 (2017) Article DOI: 10.1016/j.bmcl.2017.03.064 BindingDB Entry DOI: 10.7270/Q2SX6GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |