

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406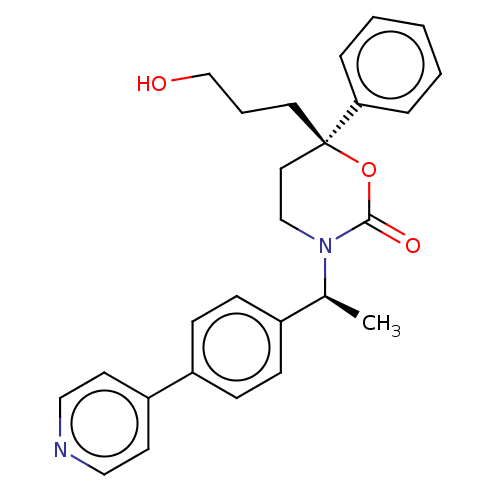 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107475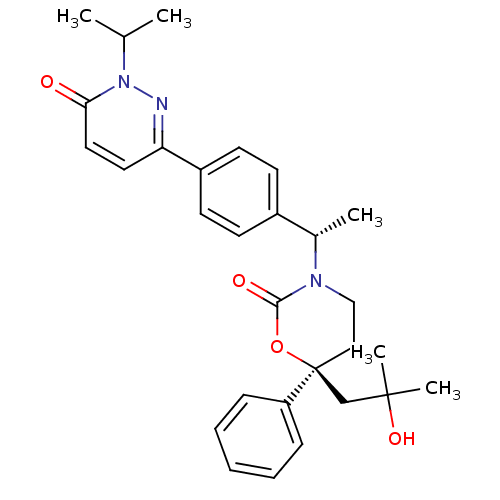 (US8592410, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514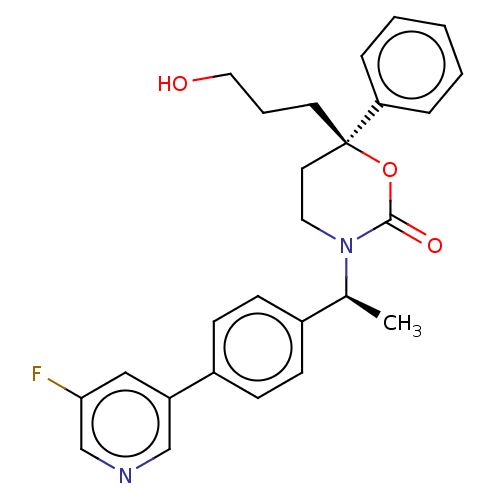 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253514 (CHEMBL4075869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353390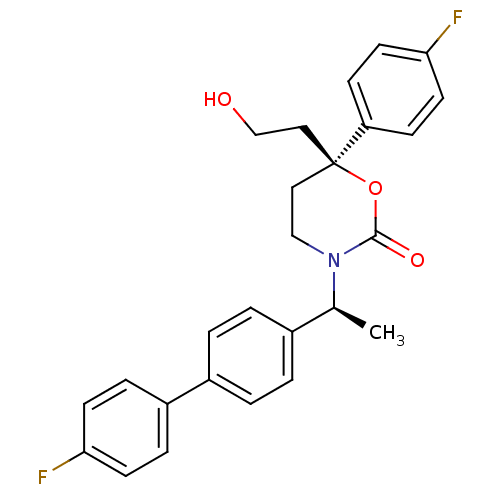 (CHEMBL1829768 | US8575157, 193 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392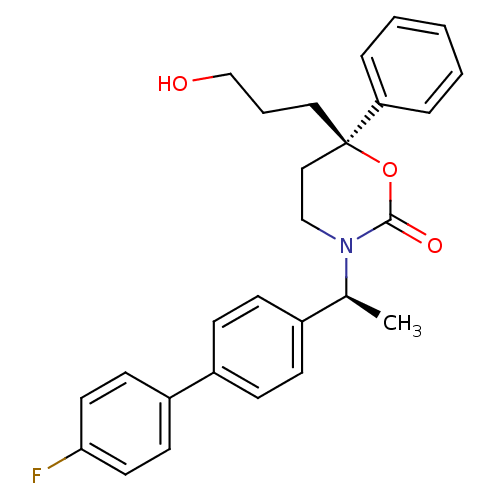 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107485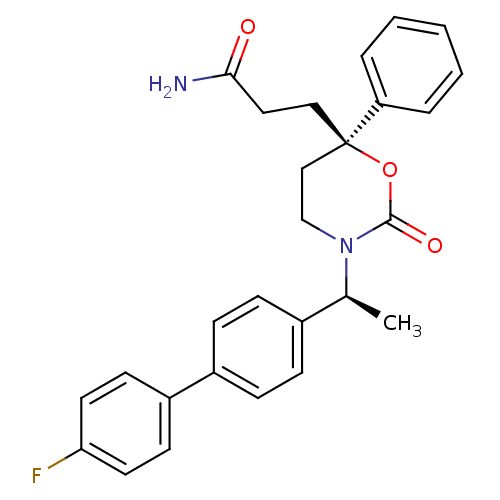 (US8575157, 199 | US8592410, Comparator 11 | US8598...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253406 (CHEMBL4101787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253398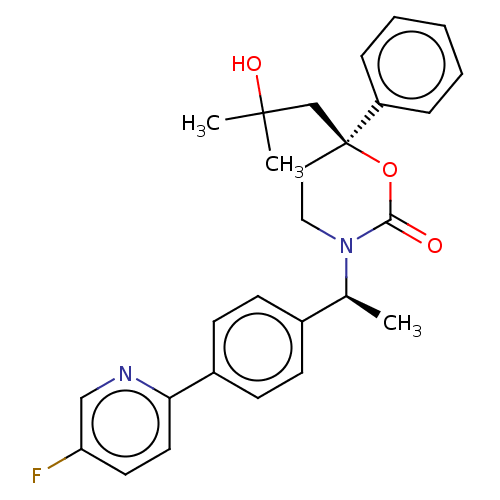 (CHEMBL4096179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253513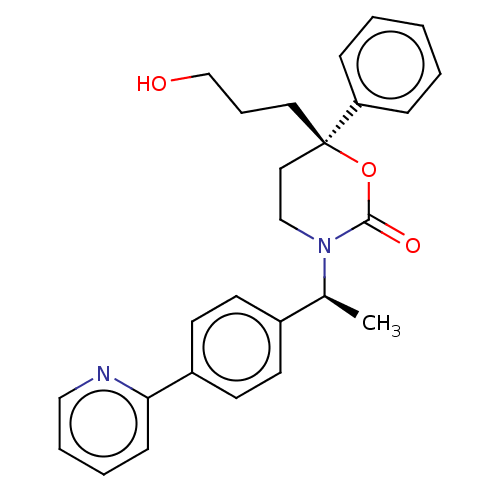 (CHEMBL4060843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253336 (CHEMBL4069717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253404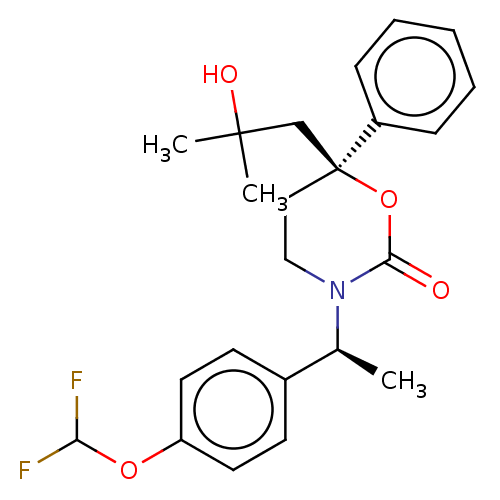 (CHEMBL4103620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253507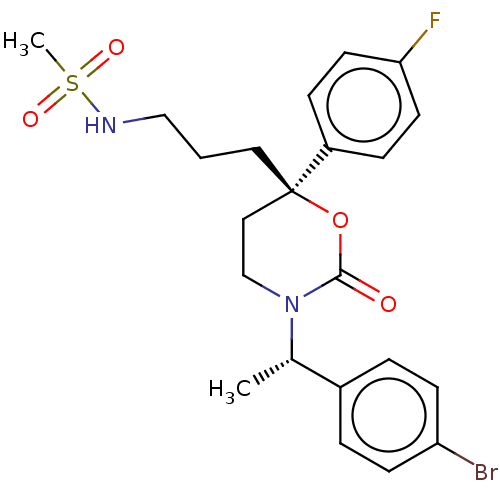 (CHEMBL4090672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253513 (CHEMBL4060843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253507 (CHEMBL4090672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353405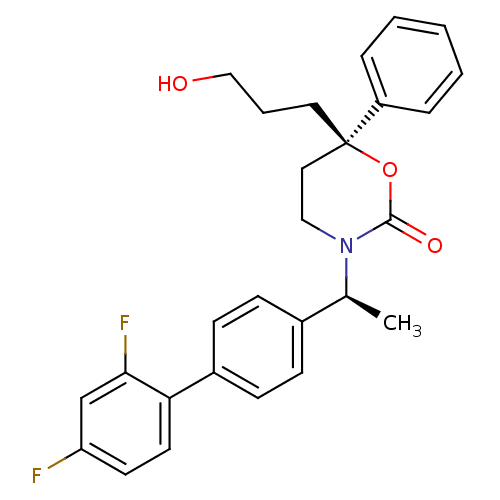 (CHEMBL1829759 | US8575157, 196 | US8592410, 93 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353386 (CHEMBL1829763 | US8592410, 88 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107487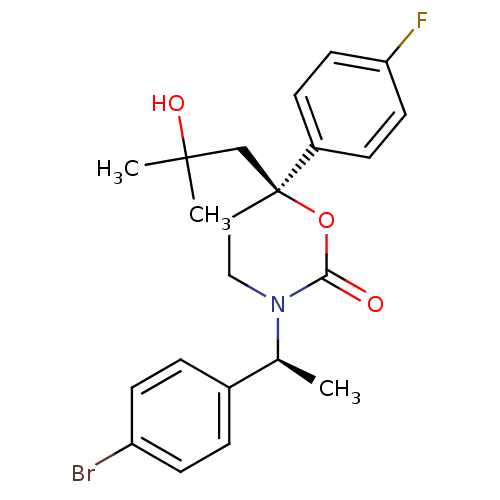 (US8575157, 201 | US8592410, Comparator 13 | US8598...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107481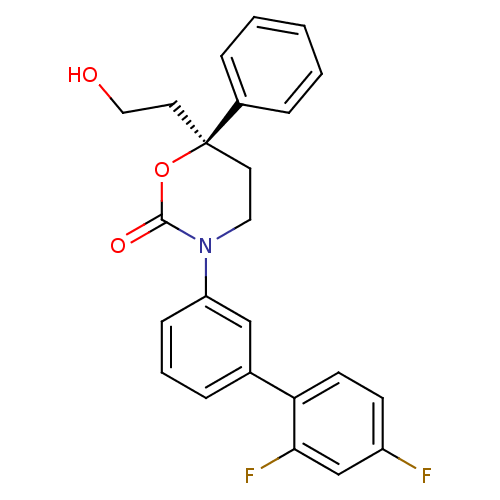 (US8575157, 189 | US8592410, Comparator 1 | US85981...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107489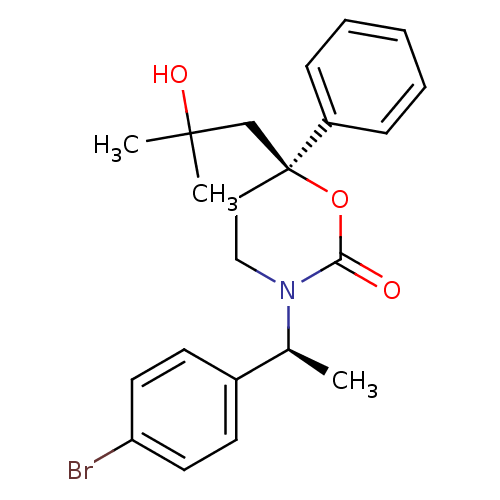 (US8575157, 203 | US8592410, Comparator 15 | US8598...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253506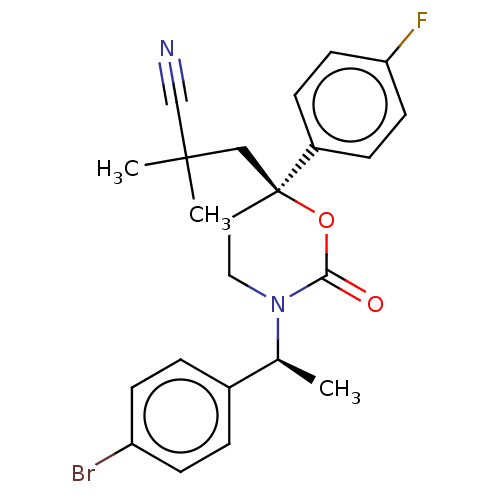 (CHEMBL4072907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253506 (CHEMBL4072907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253405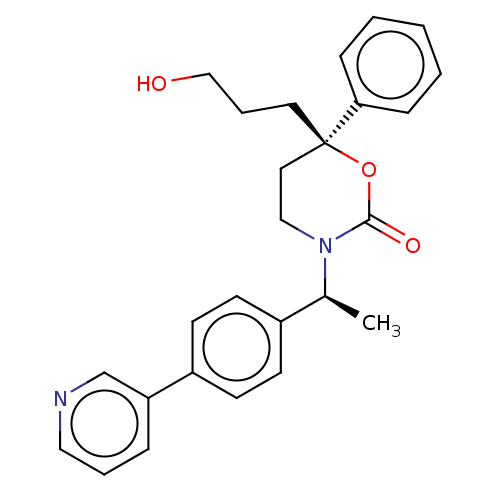 (CHEMBL4070571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253398 (CHEMBL4096179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353386 (CHEMBL1829763 | US8592410, 88 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107487 (US8575157, 201 | US8592410, Comparator 13 | US8598...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253337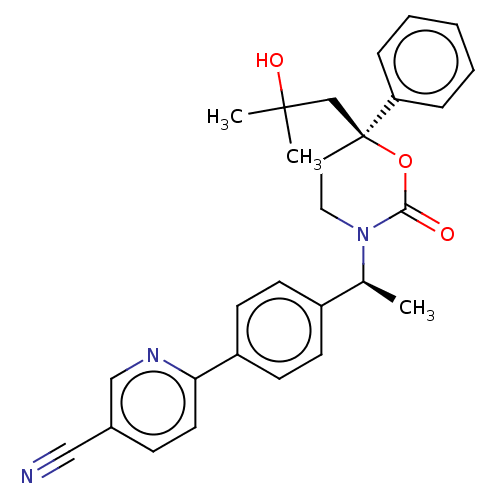 (CHEMBL4090128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107479 (US8592410, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253336 (CHEMBL4069717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human omental adipocytes using [3H]cortisone as substrate preincubated for 1 hr followed by substrate addition measured ... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107492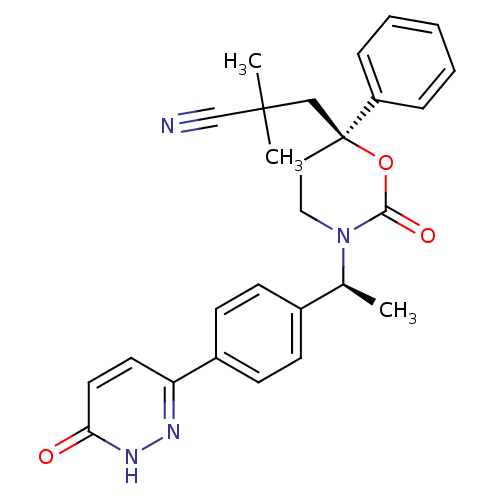 (US8592410, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107469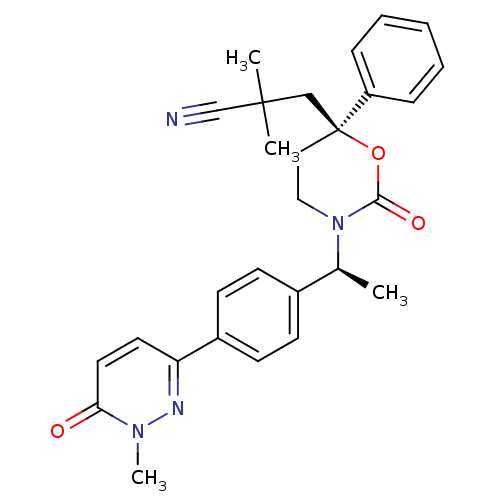 (US8592410, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353387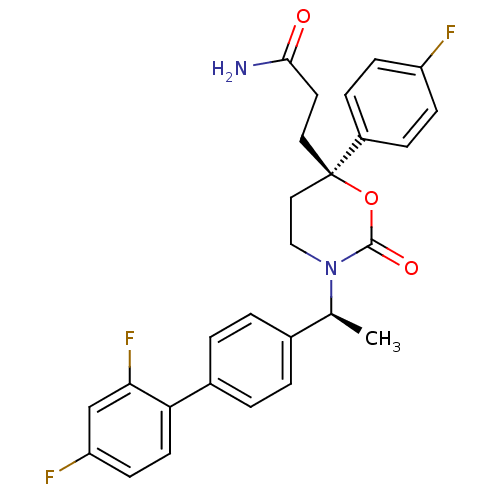 (CHEMBL1829777 | US8575157, 195 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228394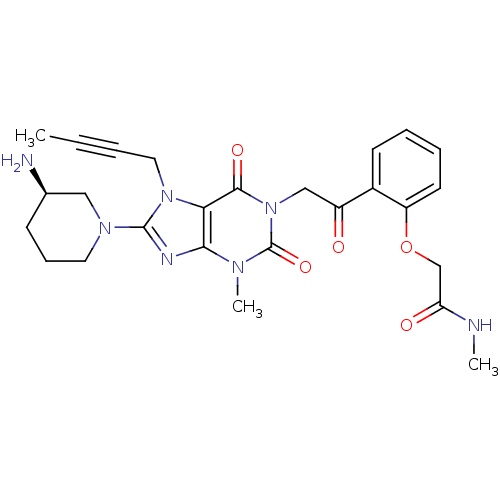 ((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of human DPP4 in Caco2 cells by fluorescene assay | J Med Chem 50: 6450-3 (2007) Article DOI: 10.1021/jm701280z BindingDB Entry DOI: 10.7270/Q2639PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224601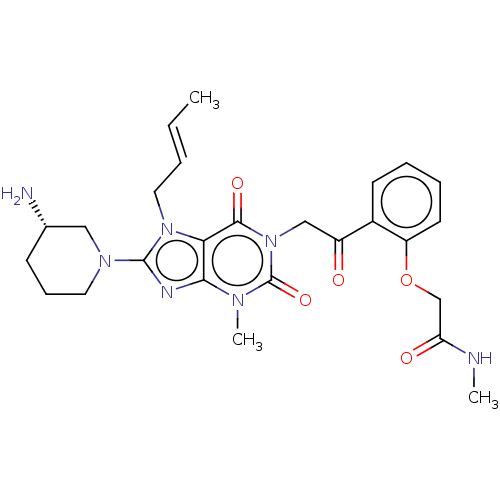 (US10202383, Example 2(150) | US9321791, 2(150) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US9556175 (2017) BindingDB Entry DOI: 10.7270/Q2Z03B5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224600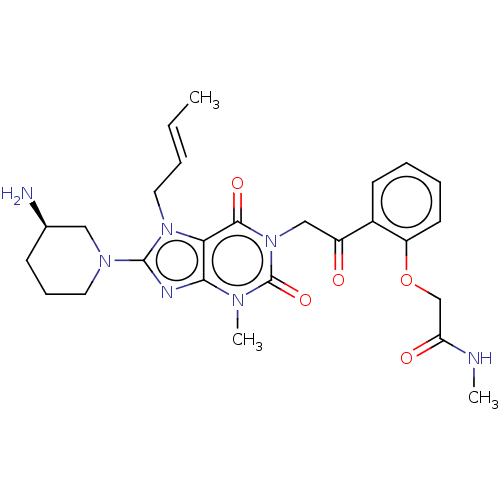 (US10202383, Example 2(148) | US9321791, 2(148) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... | US Patent US9321791 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... | US Patent US9321791 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WNR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224594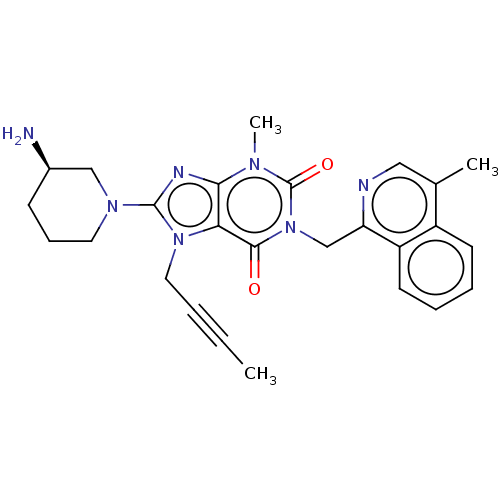 (US10202383, Example 2(132) | US9321791, 2(132) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... | US Patent US9321791 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US10202383 (2019) BindingDB Entry DOI: 10.7270/Q2P55QNN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224600 (US10202383, Example 2(148) | US9321791, 2(148) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US10202383 (2019) BindingDB Entry DOI: 10.7270/Q2P55QNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224601 (US10202383, Example 2(150) | US9321791, 2(150) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US10202383 (2019) BindingDB Entry DOI: 10.7270/Q2P55QNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228394 ((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... | US Patent US9321791 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224587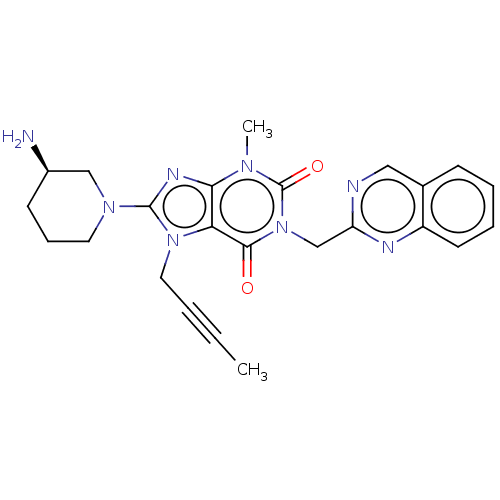 (US10202383, Example 2(80) | US9321791, 2(80) | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... | US Patent US9321791 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US9556175 (2017) BindingDB Entry DOI: 10.7270/Q2Z03B5T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50253339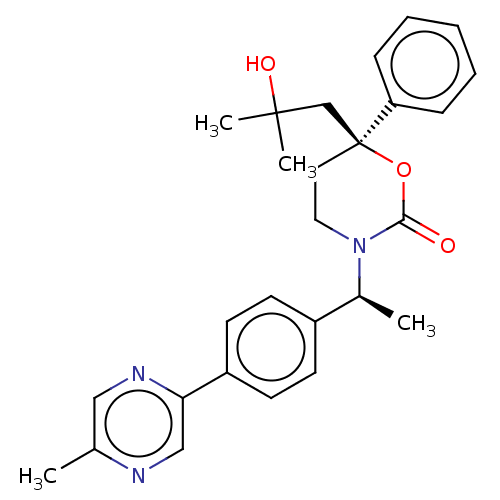 (CHEMBL4064441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Vitae Pharmaceuticals, Inc, 502 West Office Center Drive, Fort Washington, PA 19034, United States. Electronic address: linghang_zhuang@yahoo.com. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHO cell microsomes using [3H]cortisone as substrate preincubated with substrate for 10 mins followed by... | Bioorg Med Chem 25: 3649-3657 (2017) Article DOI: 10.1016/j.bmc.2017.04.033 BindingDB Entry DOI: 10.7270/Q2KS6V0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224594 (US10202383, Example 2(132) | US9321791, 2(132) | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US9556175 (2017) BindingDB Entry DOI: 10.7270/Q2Z03B5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228394 ((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US9556175 (2017) BindingDB Entry DOI: 10.7270/Q2Z03B5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM224587 (US10202383, Example 2(80) | US9321791, 2(80) | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description 50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... | US Patent US9556175 (2017) BindingDB Entry DOI: 10.7270/Q2Z03B5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376984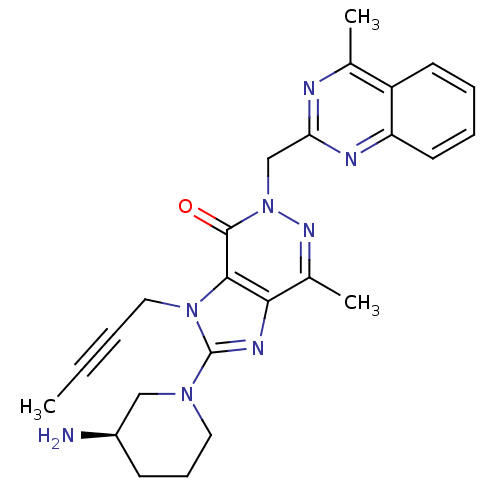 (CHEMBL256121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50376986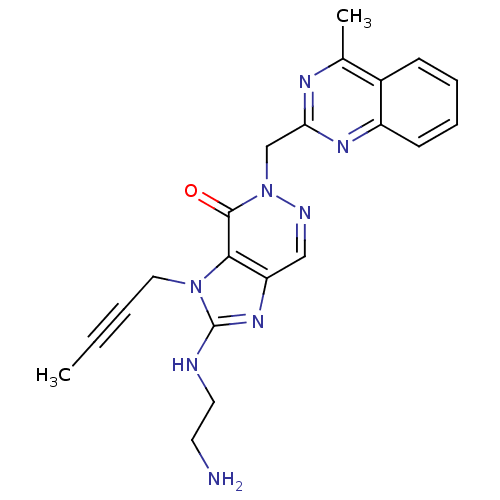 (CHEMBL257376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco-2 cells after 1 hr | Bioorg Med Chem Lett 18: 3158-62 (2008) Article DOI: 10.1016/j.bmcl.2008.04.075 BindingDB Entry DOI: 10.7270/Q2ZP470M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1690 total ) | Next | Last >> |