 Found 463 hits with Last Name = 'witt' and Initial = 'f'
Found 463 hits with Last Name = 'witt' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218750
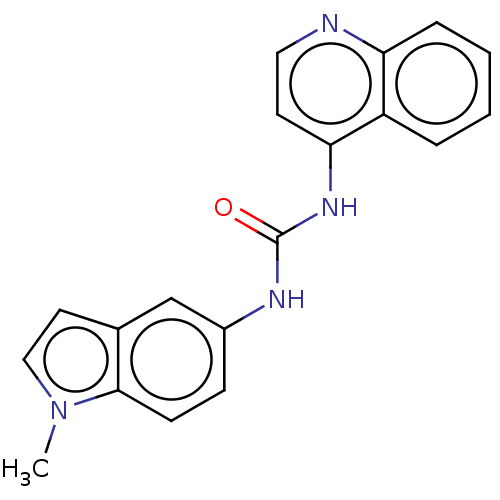
(CHEMBL299498)Show InChI InChI=1S/C19H16N4O/c1-23-11-9-13-12-14(6-7-18(13)23)21-19(24)22-17-8-10-20-16-5-3-2-4-15(16)17/h2-12H,1H3,(H2,20,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218751
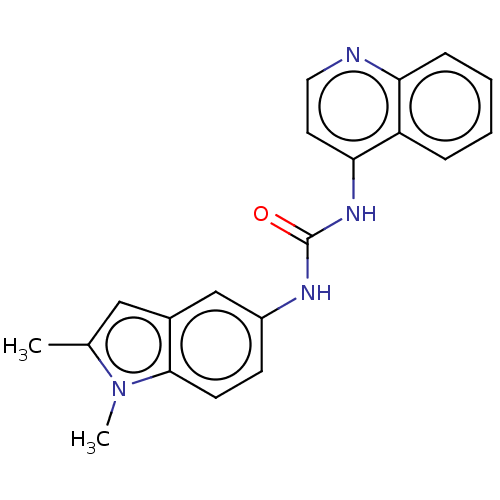
(CHEMBL300835)Show InChI InChI=1S/C20H18N4O/c1-13-11-14-12-15(7-8-19(14)24(13)2)22-20(25)23-18-9-10-21-17-6-4-3-5-16(17)18/h3-12H,1-2H3,(H2,21,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50167898
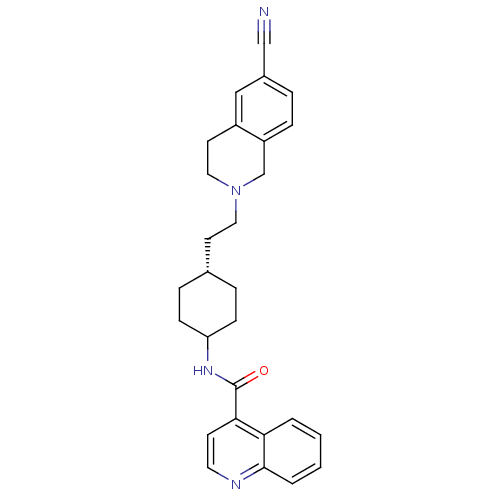
(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218748
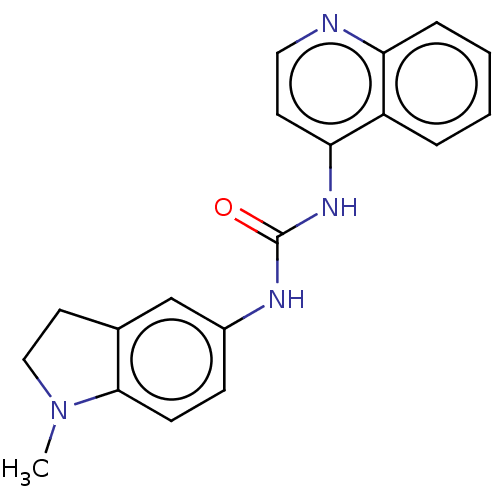
(CHEMBL301209)Show InChI InChI=1S/C19H18N4O/c1-23-11-9-13-12-14(6-7-18(13)23)21-19(24)22-17-8-10-20-16-5-3-2-4-15(16)17/h2-8,10,12H,9,11H2,1H3,(H2,20,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218752
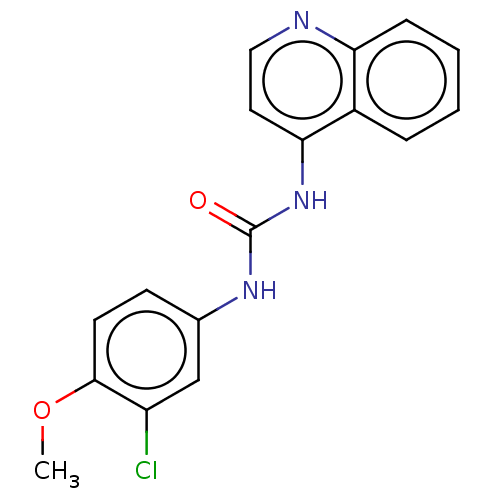
(CHEMBL56557)Show InChI InChI=1S/C17H14ClN3O2/c1-23-16-7-6-11(10-13(16)18)20-17(22)21-15-8-9-19-14-5-3-2-4-12(14)15/h2-10H,1H3,(H2,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218750

(CHEMBL299498)Show InChI InChI=1S/C19H16N4O/c1-23-11-9-13-12-14(6-7-18(13)23)21-19(24)22-17-8-10-20-16-5-3-2-4-15(16)17/h2-12H,1H3,(H2,20,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218749
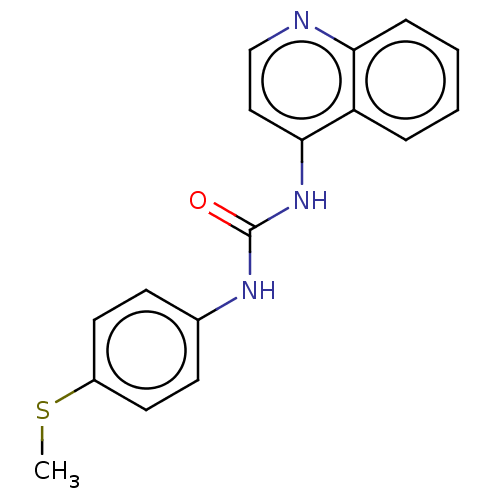
(CHEMBL54062)Show InChI InChI=1S/C17H15N3OS/c1-22-13-8-6-12(7-9-13)19-17(21)20-16-10-11-18-15-5-3-2-4-14(15)16/h2-11H,1H3,(H2,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218749

(CHEMBL54062)Show InChI InChI=1S/C17H15N3OS/c1-22-13-8-6-12(7-9-13)19-17(21)20-16-10-11-18-15-5-3-2-4-14(15)16/h2-11H,1H3,(H2,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218745
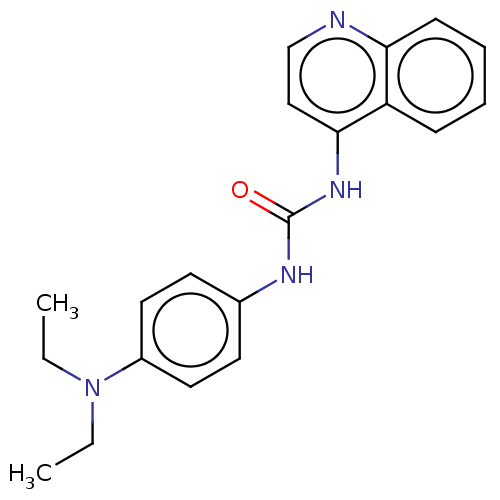
(CHEMBL57135)Show InChI InChI=1S/C20H22N4O/c1-3-24(4-2)16-11-9-15(10-12-16)22-20(25)23-19-13-14-21-18-8-6-5-7-17(18)19/h5-14H,3-4H2,1-2H3,(H2,21,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575
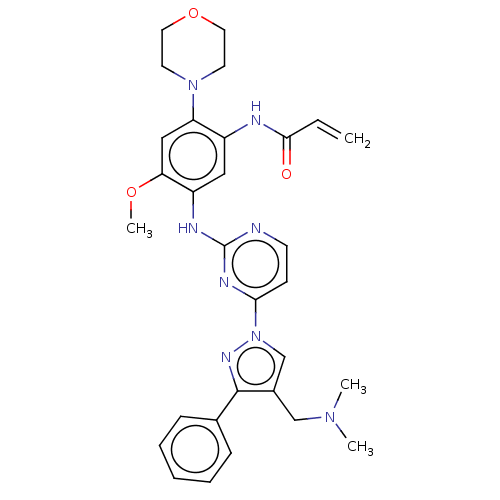
(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218752

(CHEMBL56557)Show InChI InChI=1S/C17H14ClN3O2/c1-23-16-7-6-11(10-13(16)18)20-17(22)21-15-8-9-19-14-5-3-2-4-12(14)15/h2-10H,1H3,(H2,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218745

(CHEMBL57135)Show InChI InChI=1S/C20H22N4O/c1-3-24(4-2)16-11-9-15(10-12-16)22-20(25)23-19-13-14-21-18-8-6-5-7-17(18)19/h5-14H,3-4H2,1-2H3,(H2,21,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218748

(CHEMBL301209)Show InChI InChI=1S/C19H18N4O/c1-23-11-9-13-12-14(6-7-18(13)23)21-19(24)22-17-8-10-20-16-5-3-2-4-15(16)17/h2-8,10,12H,9,11H2,1H3,(H2,20,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218751

(CHEMBL300835)Show InChI InChI=1S/C20H18N4O/c1-13-11-14-12-15(7-8-19(14)24(13)2)22-20(25)23-18-9-10-21-17-6-4-3-5-16(17)18/h3-12H,1-2H3,(H2,21,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218747
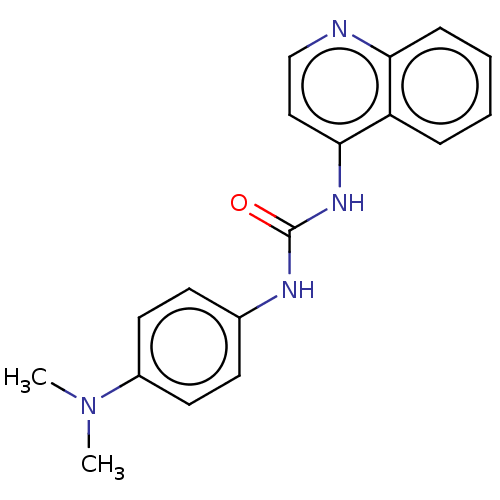
(CHEMBL417753)Show InChI InChI=1S/C18H18N4O/c1-22(2)14-9-7-13(8-10-14)20-18(23)21-17-11-12-19-16-6-4-3-5-15(16)17/h3-12H,1-2H3,(H2,19,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218747

(CHEMBL417753)Show InChI InChI=1S/C18H18N4O/c1-22(2)14-9-7-13(8-10-14)20-18(23)21-17-11-12-19-16-6-4-3-5-15(16)17/h3-12H,1-2H3,(H2,19,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 434 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50218746
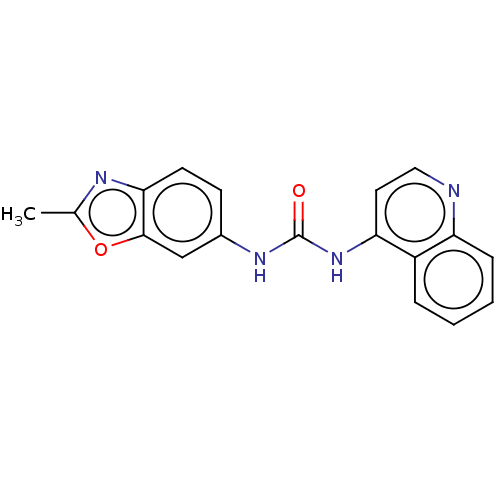
(CHEMBL59266)Show InChI InChI=1S/C18H14N4O2/c1-11-20-16-7-6-12(10-17(16)24-11)21-18(23)22-15-8-9-19-14-5-3-2-4-13(14)15/h2-10H,1H3,(H2,19,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50218746

(CHEMBL59266)Show InChI InChI=1S/C18H14N4O2/c1-11-20-16-7-6-12(10-17(16)24-11)21-18(23)22-15-8-9-19-14-5-3-2-4-13(14)15/h2-10H,1H3,(H2,19,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50384416

(CHEMBL2111553 | CHEMBL291536 | SB-334867)Show InChI InChI=1S/C17H13N5O2/c1-10-20-12-5-4-11(9-15(12)24-10)21-17(23)22-14-6-8-18-13-3-2-7-19-16(13)14/h2-9H,1H3,(H2,18,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2B receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50384416

(CHEMBL2111553 | CHEMBL291536 | SB-334867)Show InChI InChI=1S/C17H13N5O2/c1-10-20-12-5-4-11(9-15(12)24-10)21-17(23)22-14-6-8-18-13-3-2-7-19-16(13)14/h2-9H,1H3,(H2,18,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| <5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 11: 1907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2G16318 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50167898

(CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydro...)Show SMILES O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 294: 1154-65 (2000)
BindingDB Entry DOI: 10.7270/Q2K35S7G |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546980

(CHEMBL4792513)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(F)C2CCOC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R/T790M mutant using poly[Glu:Tyr] (4:1) as substrate measured in presence of [gamma-33P]ATP by radiometric assay |
J Med Chem 63: 4293-4305 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00200
BindingDB Entry DOI: 10.7270/Q2J38WXH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50514823
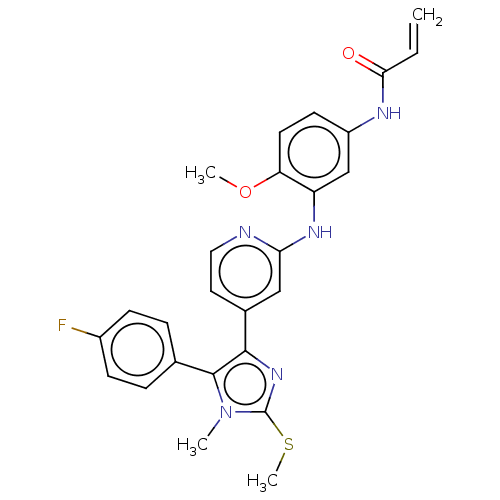
(CHEMBL4575583)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1nc(SC)n(C)c1-c1ccc(F)cc1 Show InChI InChI=1S/C26H24FN5O2S/c1-5-23(33)29-19-10-11-21(34-3)20(15-19)30-22-14-17(12-13-28-22)24-25(32(2)26(31-24)35-4)16-6-8-18(27)9-7-16/h5-15H,1H2,2-4H3,(H,28,30)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R/T790M mutant using poly[Glu:Tyr] (4:1) as substrate measured in presence of [gamma-33P]ATP by radiometric assay |
J Med Chem 63: 4293-4305 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00200
BindingDB Entry DOI: 10.7270/Q2J38WXH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602487
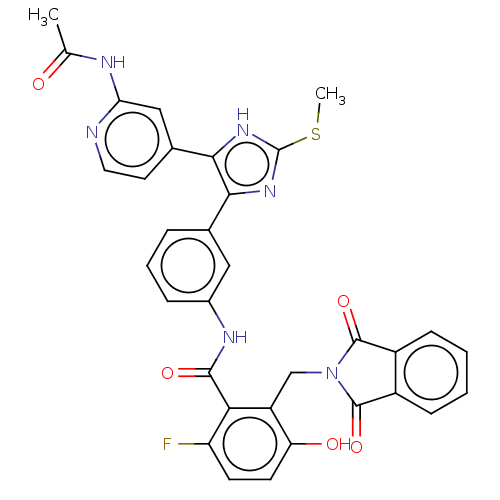
(CHEMBL5183286)Show SMILES CSc1nc(c([nH]1)-c1ccnc(NC(C)=O)c1)-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602490
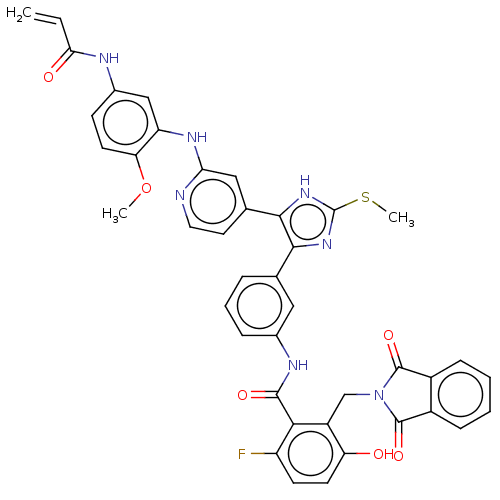
(CHEMBL5188399)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546969
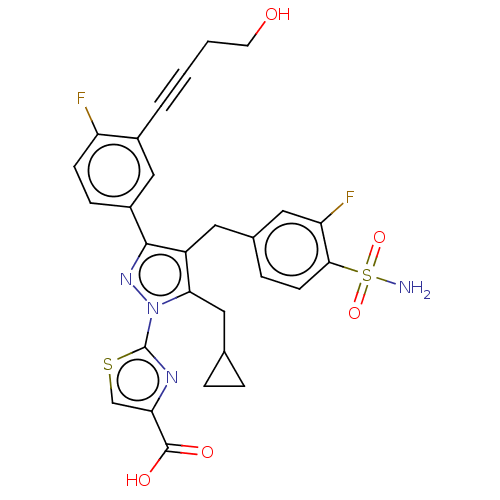
(CHEMBL4786682 | US11247971, Cmpd ID 409)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CCCO)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602490

(CHEMBL5188399)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50546981

(CHEMBL4797357)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)C#CC(O)C2CCOC2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00916
BindingDB Entry DOI: 10.7270/Q2057KJ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data