 Found 267 hits with Last Name = 'wuest' and Initial = 'f'
Found 267 hits with Last Name = 'wuest' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50319376
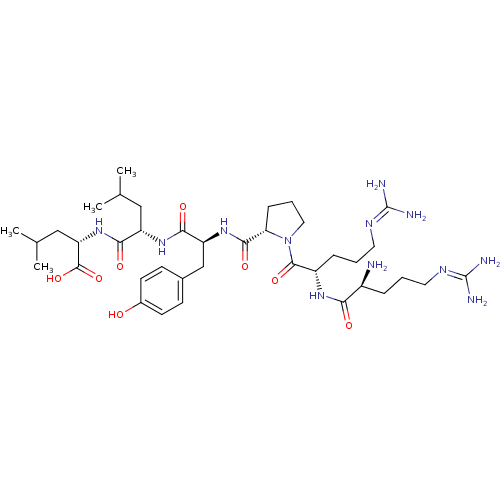
((S)-2-((S)-2-((S)-2-((S)-1-((S)-2-((S)-2-amino-5-g...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-21(2)18-27(32(53)49-29(36(57)58)19-22(3)4)47-33(54)28(20-23-11-13-24(51)14-12-23)48-34(55)30-10-7-17-50(30)35(56)26(9-6-16-45-38(42)43)46-31(52)25(39)8-5-15-44-37(40)41/h11-14,21-22,25-30,51H,5-10,15-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t25-,26-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Displacement of [3H]neurotensin from NTR1 in human HT29 cells after 30 mins by liquid scintillation spectrometry |
Bioorg Med Chem Lett 20: 3306-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.038
BindingDB Entry DOI: 10.7270/Q2V40VC1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50376383
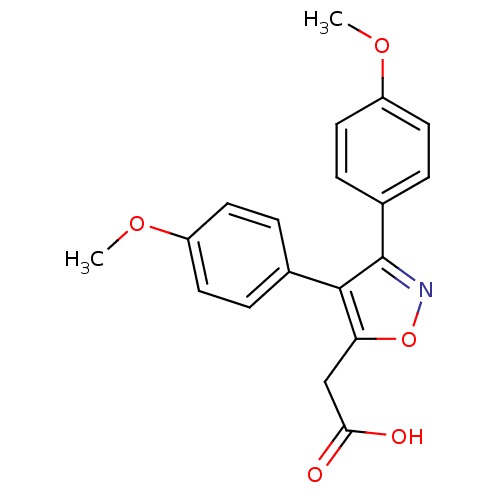
(CHEMBL259972)Show InChI InChI=1S/C19H17NO5/c1-23-14-7-3-12(4-8-14)18-16(11-17(21)22)25-20-19(18)13-5-9-15(24-2)10-6-13/h3-10H,11H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029618

(4-(2-(4-methoxyphenyl)cyclopent-1-enyl)benzenesulf...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H19NO3S/c1-22-15-9-5-13(6-10-15)17-3-2-4-18(17)14-7-11-16(12-8-14)23(19,20)21/h5-12H,2-4H2,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 16: 7662-70 (2008)
Article DOI: 10.1016/j.bmc.2008.07.016
BindingDB Entry DOI: 10.7270/Q2K64HXS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029613
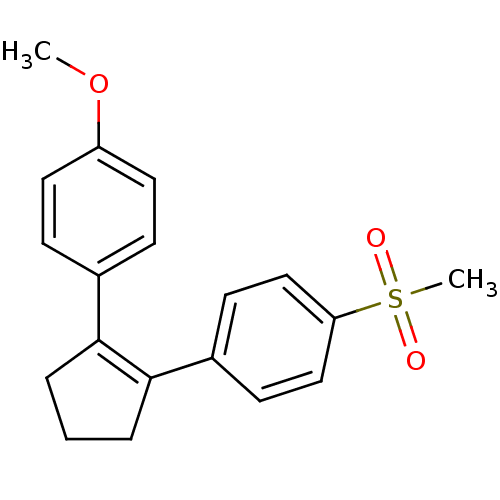
(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 16: 7662-70 (2008)
Article DOI: 10.1016/j.bmc.2008.07.016
BindingDB Entry DOI: 10.7270/Q2K64HXS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50084631

(CHEMBL3427203)Show InChI InChI=1S/C17H17N3O3/c1-21-14-8-4-12(5-9-14)16-18-17(23-3)19-20(16)13-6-10-15(22-2)11-7-13/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095
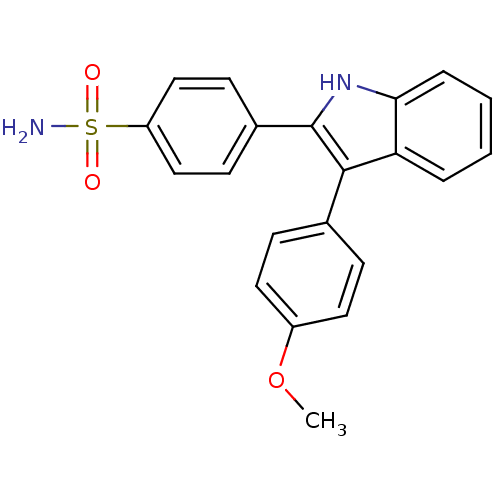
(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 20: 3410-21 (2012)
Article DOI: 10.1016/j.bmc.2012.04.022
BindingDB Entry DOI: 10.7270/Q2RF5W3X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297675
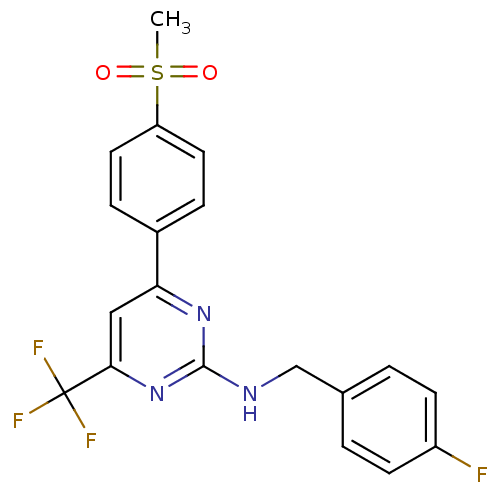
(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029613

(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13065

(5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C17H12ClF3N2O/c1-24-14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 in human OVCAR-3 cells assessed as reduction in PGE2 level incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50096276
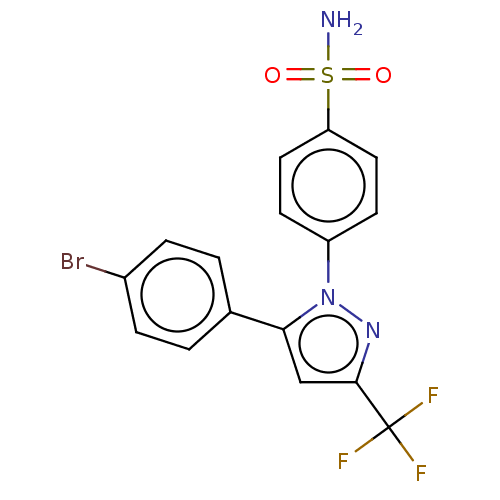
(CHEMBL1235806)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Br)cc1)C(F)(F)F Show InChI InChI=1S/C12H6N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H2,13,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM13065

(5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C17H12ClF3N2O/c1-24-14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant ovine COX1 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50366159
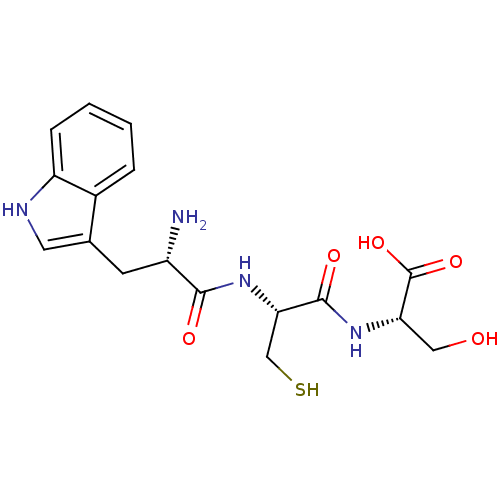
(CHEMBL1957450)Show SMILES N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O |r| Show InChI InChI=1S/C17H22N4O5S/c18-11(5-9-6-19-12-4-2-1-3-10(9)12)15(23)21-14(8-27)16(24)20-13(7-22)17(25)26/h1-4,6,11,13-14,19,22,27H,5,7-8,18H2,(H,20,24)(H,21,23)(H,25,26)/t11-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by ELISA |
Bioorg Med Chem 20: 2221-6 (2012)
Article DOI: 10.1016/j.bmc.2012.02.021
BindingDB Entry DOI: 10.7270/Q2RV0P5K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Madaba
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as PGH2 formation preincubated for 10 mins followed by addition of arachidonic acid as substrate measur... |
Bioorg Med Chem Lett 26: 4757-4762 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.034
BindingDB Entry DOI: 10.7270/Q2VT1WKD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 by fluorescence assay |
Bioorg Med Chem 21: 4288-95 (2013)
Article DOI: 10.1016/j.bmc.2013.04.074
BindingDB Entry DOI: 10.7270/Q2DV1NTZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272096
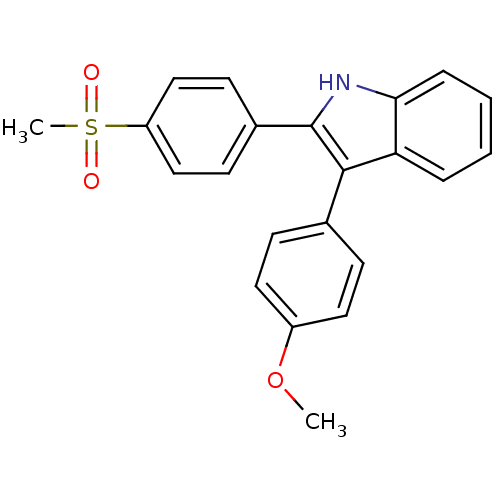
(3-(4-methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)-1...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO3S/c1-26-17-11-7-15(8-12-17)21-19-5-3-4-6-20(19)23-22(21)16-9-13-18(14-10-16)27(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 20: 3410-21 (2012)
Article DOI: 10.1016/j.bmc.2012.04.022
BindingDB Entry DOI: 10.7270/Q2RF5W3X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272131

(3-(4-fluorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(F)cc1 Show InChI InChI=1S/C21H16FNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem 20: 3410-21 (2012)
Article DOI: 10.1016/j.bmc.2012.04.022
BindingDB Entry DOI: 10.7270/Q2RF5W3X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266516
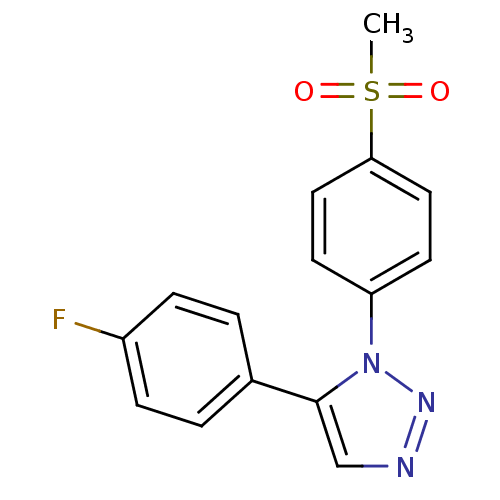
(5-(4-Fluorophenyl)-1-(4-methanesulfonylphenyl)-1H-...)Show InChI InChI=1S/C15H12FN3O2S/c1-22(20,21)14-8-6-13(7-9-14)19-15(10-17-18-19)11-2-4-12(16)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 by fluorescence assay |
Bioorg Med Chem Lett 21: 1823-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.057
BindingDB Entry DOI: 10.7270/Q2KD1Z6P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266515
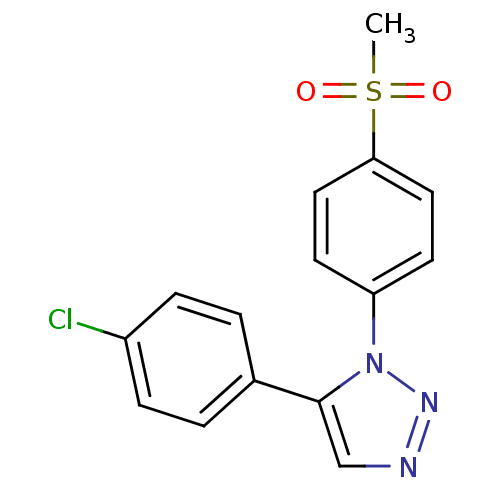
(5-(4-Chlorophenyl)-1-(4-methanesulfonylphenyl)-1H-...)Show InChI InChI=1S/C15H12ClN3O2S/c1-22(20,21)14-8-6-13(7-9-14)19-15(10-17-18-19)11-2-4-12(16)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 preincubated for 5 mins followed by addition of arachidonic acid as substrate measured after 2 mins by fluoresc... |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 by fluorescence assay |
Bioorg Med Chem 20: 2221-6 (2012)
Article DOI: 10.1016/j.bmc.2012.02.021
BindingDB Entry DOI: 10.7270/Q2RV0P5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057564
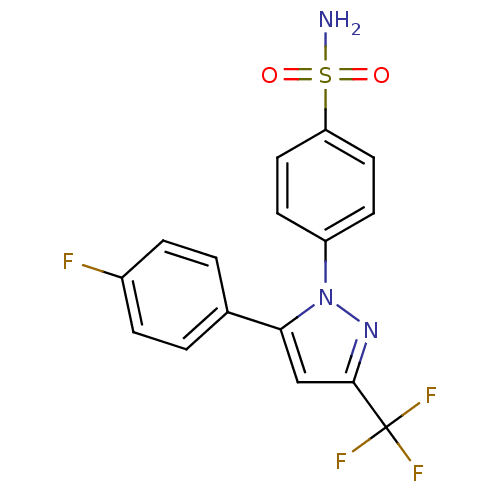
(4-[5-(4-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant COX2 by enzyme immunoassay |
Bioorg Med Chem Lett 22: 2235-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.093
BindingDB Entry DOI: 10.7270/Q2S182ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50207446
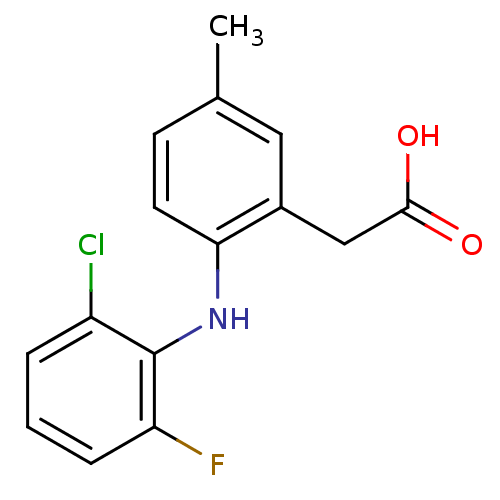
(2-(2-(2-chloro-6-fluorophenylamino)-5-methylphenyl...)Show InChI InChI=1S/C15H13ClFNO2/c1-9-5-6-13(10(7-9)8-14(19)20)18-15-11(16)3-2-4-12(15)17/h2-7,18H,8H2,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Bioorg Med Chem Lett 26: 1516-20 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.029
BindingDB Entry DOI: 10.7270/Q2377BKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 by enzymatic fluorescence assay |
Bioorg Med Chem 20: 3410-21 (2012)
Article DOI: 10.1016/j.bmc.2012.04.022
BindingDB Entry DOI: 10.7270/Q2RF5W3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50557782
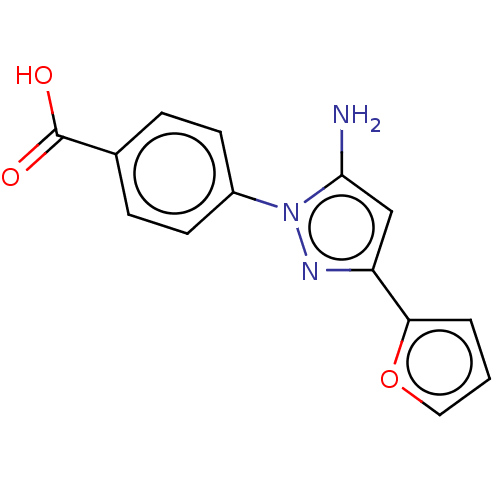
(CHEMBL4800072) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 in human OVCAR-3 cells assessed as reduction in PGE2 level incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
Bioorg Med Chem Lett 23: 163-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.131
BindingDB Entry DOI: 10.7270/Q26T0NZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50557783
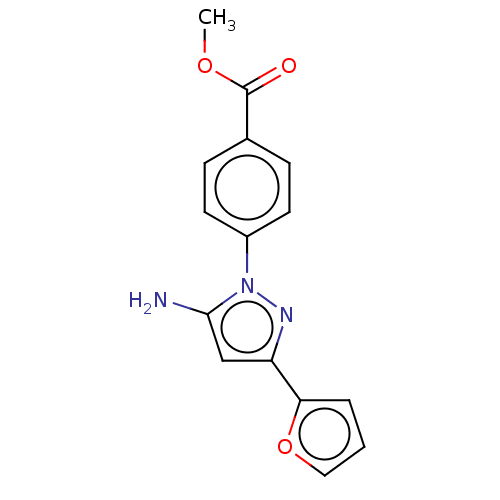
(CHEMBL4784073) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 in human OVCAR-3 cells assessed as reduction in PGE2 level incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50557782

(CHEMBL4800072) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant ovine COX1 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266511
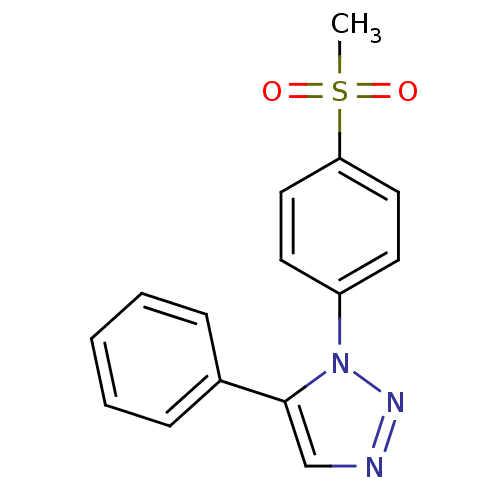
(1-(4-Methanesulfonylphenyl)-5-phenyl-1H-[1,2,3]tri...)Show InChI InChI=1S/C15H13N3O2S/c1-21(19,20)14-9-7-13(8-10-14)18-15(11-16-17-18)12-5-3-2-4-6-12/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4810
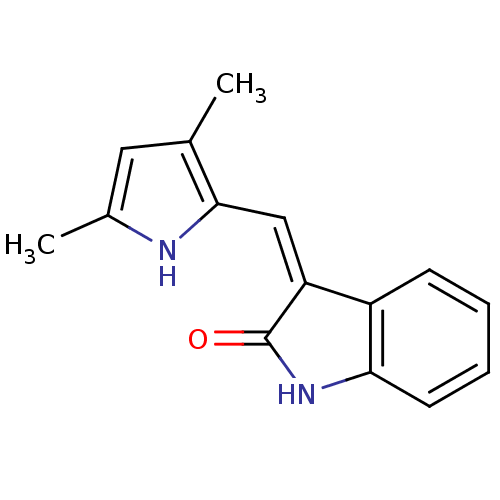
((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of FLK1 |
Bioorg Med Chem 17: 7732-42 (2009)
Article DOI: 10.1016/j.bmc.2009.09.038
BindingDB Entry DOI: 10.7270/Q2PV6KFK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266512
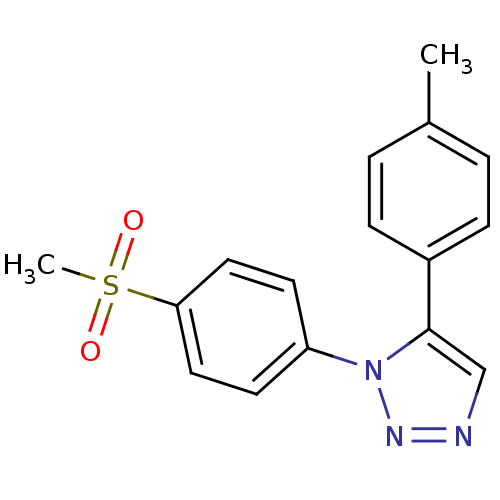
(1-(4-Methanesulfonyl-phenyl)-5-p-tolyl-1H-[1,2,3]t...)Show InChI InChI=1S/C16H15N3O2S/c1-12-3-5-13(6-4-12)16-11-17-18-19(16)14-7-9-15(10-8-14)22(2,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266480
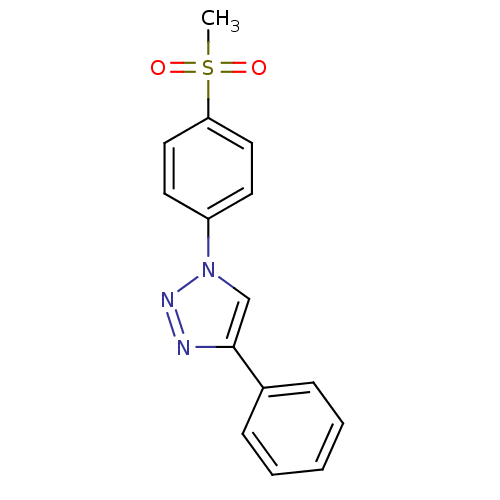
(1-(4-Methanesulfonylphenyl)-4-phenyl-1H-[1,2,3]tri...)Show InChI InChI=1S/C15H13N3O2S/c1-21(19,20)14-9-7-13(8-10-14)18-11-15(16-17-18)12-5-3-2-4-6-12/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50492260
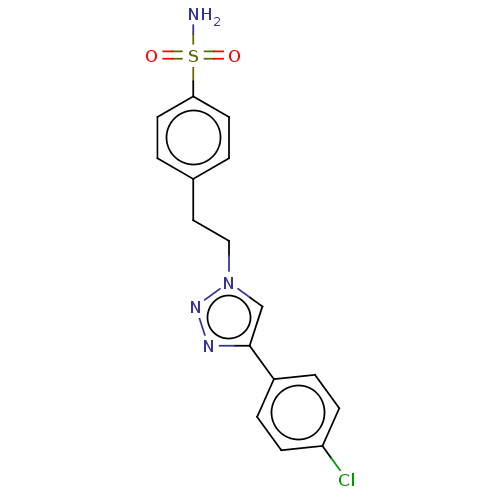
(CHEMBL2398541)Show SMILES NS(=O)(=O)c1ccc(CCn2cc(nn2)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C16H15ClN4O2S/c17-14-5-3-13(4-6-14)16-11-21(20-19-16)10-9-12-1-7-15(8-2-12)24(18,22)23/h1-8,11H,9-10H2,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 by fluorescence assay |
Bioorg Med Chem 21: 4288-95 (2013)
Article DOI: 10.1016/j.bmc.2013.04.074
BindingDB Entry DOI: 10.7270/Q2DV1NTZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266513
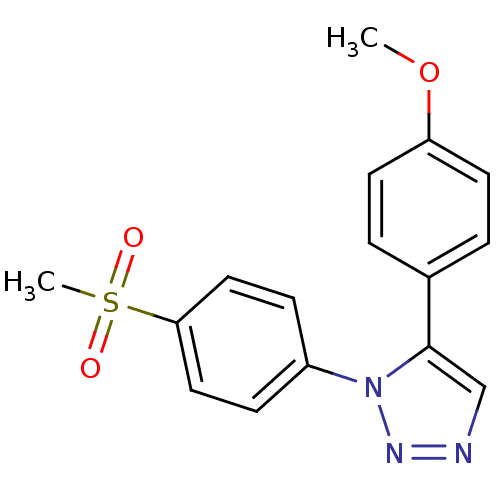
(5-(4-Methoxyphenyl)-1-(4-(methylsulfonyl)phenyl)-1...)Show InChI InChI=1S/C16H15N3O3S/c1-22-14-7-3-12(4-8-14)16-11-17-18-19(16)13-5-9-15(10-6-13)23(2,20)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50339185
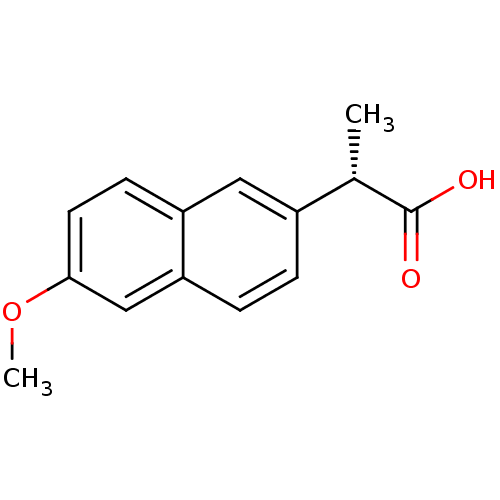
((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 23: 163-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.131
BindingDB Entry DOI: 10.7270/Q26T0NZ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266484
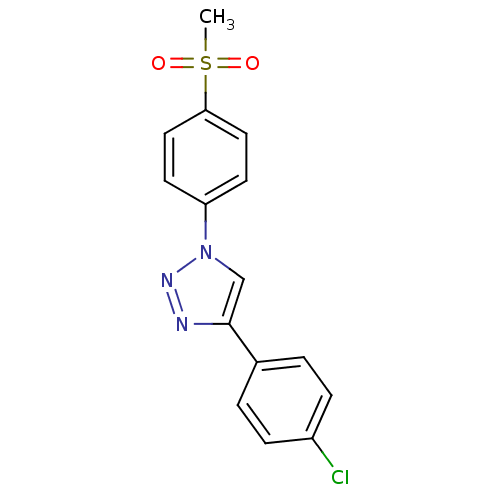
(4-(4-Chlorophenyl)-1-(4-methanesulfonylphenyl)-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1cc(nn1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClN3O2S/c1-22(20,21)14-8-6-13(7-9-14)19-10-15(17-18-19)11-2-4-12(16)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50557783

(CHEMBL4784073) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant ovine COX1 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50029618

(4-(2-(4-methoxyphenyl)cyclopent-1-enyl)benzenesulf...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H19NO3S/c1-22-15-9-5-13(6-10-15)17-3-2-4-18(17)14-7-11-16(12-8-14)23(19,20)21/h5-12H,2-4H2,1H3,(H2,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem 16: 7662-70 (2008)
Article DOI: 10.1016/j.bmc.2008.07.016
BindingDB Entry DOI: 10.7270/Q2K64HXS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266485
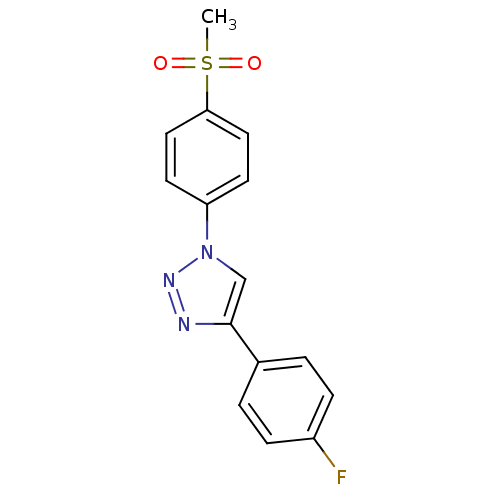
(4-(4-Fluorophenyl)-1-(4-methanesulfonylphenyl)-1H-...)Show InChI InChI=1S/C15H12FN3O2S/c1-22(20,21)14-8-6-13(7-9-14)19-10-15(17-18-19)11-2-4-12(16)5-3-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50557784
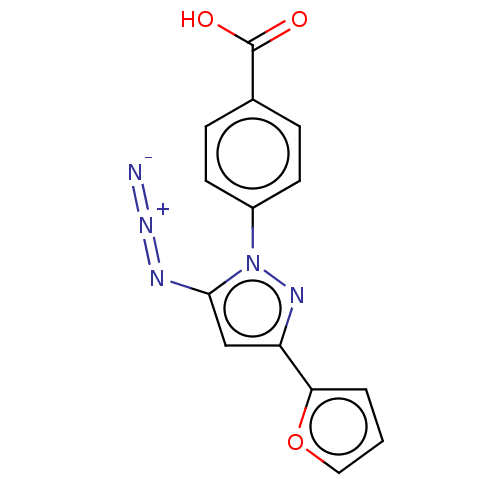
(CHEMBL4797035)Show SMILES OC(=O)c1ccc(cc1)-n1nc(cc1N=[N+]=[N-])-c1ccco1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant ovine COX1 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM22360
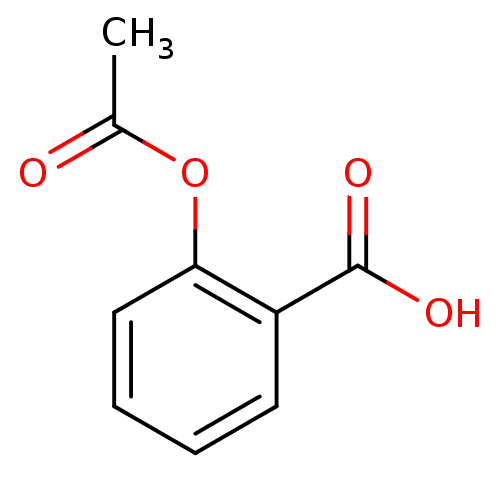
(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM22360

(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Bioorg Med Chem Lett 23: 163-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.131
BindingDB Entry DOI: 10.7270/Q26T0NZ5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM6383
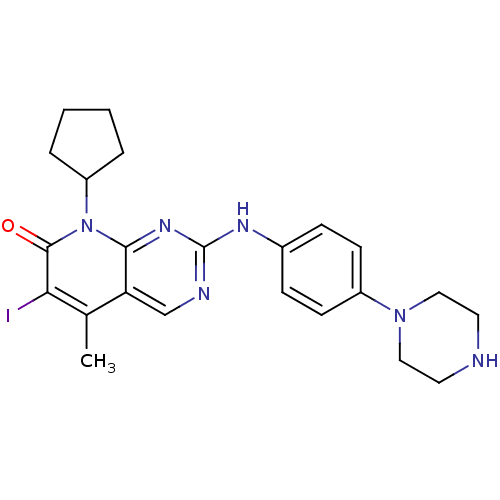
(8-Cyclopentyl-6-iodo-5-methyl-2-(4-piperazin-1-yl-...)Show SMILES Cc1c(I)c(=O)n(C2CCCC2)c2nc(Nc3ccc(cc3)N3CCNCC3)ncc12 Show InChI InChI=1S/C23H27IN6O/c1-15-19-14-26-23(27-16-6-8-17(9-7-16)29-12-10-25-11-13-29)28-21(19)30(22(31)20(15)24)18-4-2-3-5-18/h6-9,14,18,25H,2-5,10-13H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Eur J Med Chem 45: 727-37 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.020
BindingDB Entry DOI: 10.7270/Q20C4VT1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50266514
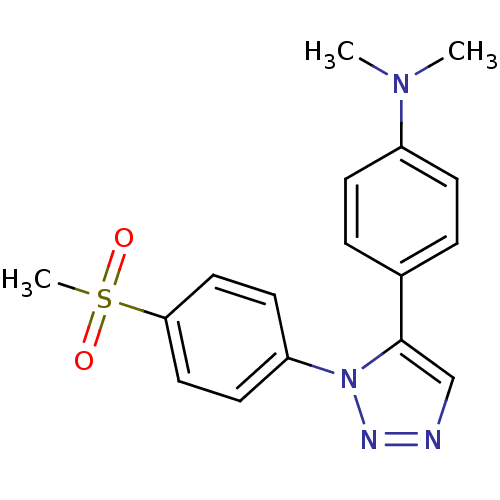
(CHEMBL457331 | N,N-dimethyl-4-(1-(4-(methylsulfony...)Show SMILES CN(C)c1ccc(cc1)-c1cnnn1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H18N4O2S/c1-20(2)14-6-4-13(5-7-14)17-12-18-19-21(17)15-8-10-16(11-9-15)24(3,22)23/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by enzyme immunoassay |
Bioorg Med Chem 17: 1146-51 (2009)
Article DOI: 10.1016/j.bmc.2008.12.032
BindingDB Entry DOI: 10.7270/Q2HQ3ZSJ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM6383

(8-Cyclopentyl-6-iodo-5-methyl-2-(4-piperazin-1-yl-...)Show SMILES Cc1c(I)c(=O)n(C2CCCC2)c2nc(Nc3ccc(cc3)N3CCNCC3)ncc12 Show InChI InChI=1S/C23H27IN6O/c1-15-19-14-26-23(27-16-6-8-17(9-7-16)29-12-10-25-11-13-29)28-21(19)30(22(31)20(15)24)18-4-2-3-5-18/h6-9,14,18,25H,2-5,10-13H2,1H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmacy
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Eur J Med Chem 45: 727-37 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.020
BindingDB Entry DOI: 10.7270/Q20C4VT1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50557784

(CHEMBL4797035)Show SMILES OC(=O)c1ccc(cc1)-n1nc(cc1N=[N+]=[N-])-c1ccco1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 in human OVCAR-3 cells assessed as reduction in PGE2 level incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00065
BindingDB Entry DOI: 10.7270/Q2988BP9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data