 Found 99 hits with Last Name = 'khan' and Initial = 'fa'
Found 99 hits with Last Name = 'khan' and Initial = 'fa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50398388
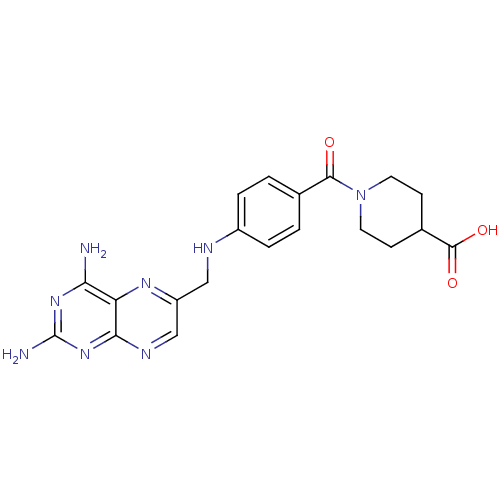
(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398388

(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562453
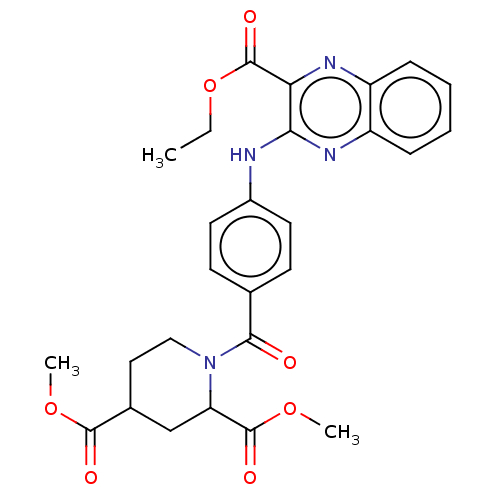
(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562451
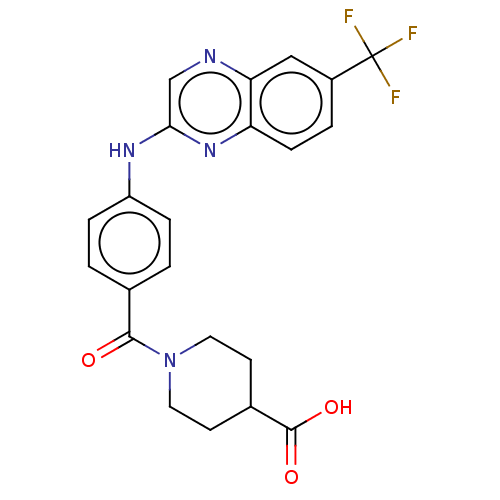
(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562452

(CHEMBL4776197)Show SMILES Nc1cc(cc2nc(c(Nc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)nc12)-c1ccccc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562451

(CHEMBL4763597)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1ccc(Nc2cnc3cc(ccc3n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562453

(CHEMBL4783402)Show SMILES CCOC(=O)c1nc2ccccc2nc1Nc1ccc(cc1)C(=O)N1CCC(CC1C(=O)OC)C(=O)OC | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR assessed by NADPH oxidation measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562445

(CHEMBL4748139)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562454

(CHEMBL533684 | TCMDC-141974) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562450
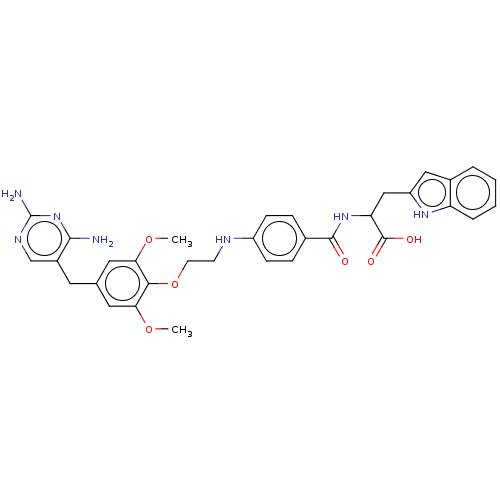
(CHEMBL4757974)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1cc2ccccc2[nH]1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562443

(CHEMBL4759800)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(C(C)C)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562444

(CHEMBL4783671)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1ccccc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562449
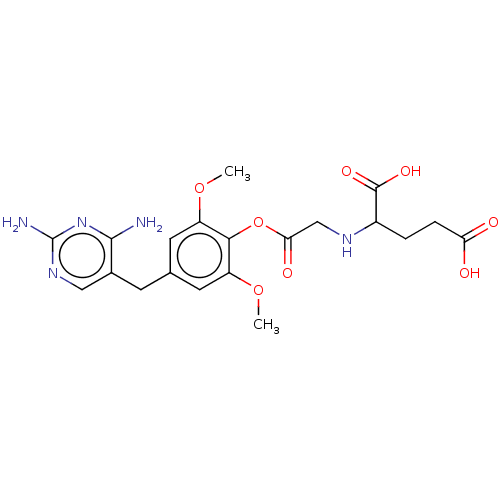
(CHEMBL4745475)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562446
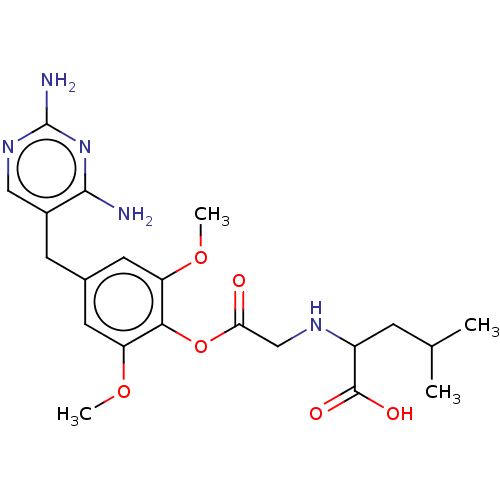
(CHEMBL4752301)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CC(C)C)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562448

(CHEMBL4779765)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1ccccc1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562447
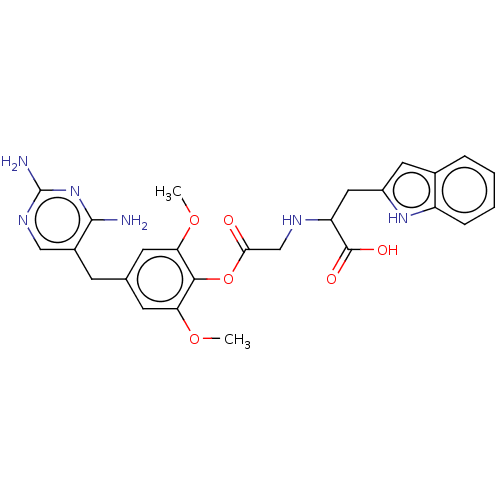
(CHEMBL4761589)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1cc2ccccc2[nH]1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562442
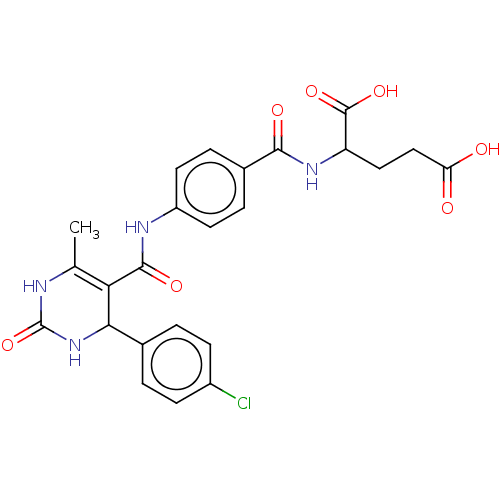
(CHEMBL4748158)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(Cl)cc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562445

(CHEMBL4748139)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562441

(CHEMBL4791810)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(cc1)[N+]([O-])=O)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562455

(CHEMBL2005041)Show SMILES Nc1nc(N)c(CCCCc2ccccc2)c(\C=C\c2ccc(cc2)[N+]([O-])=O)n1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562438
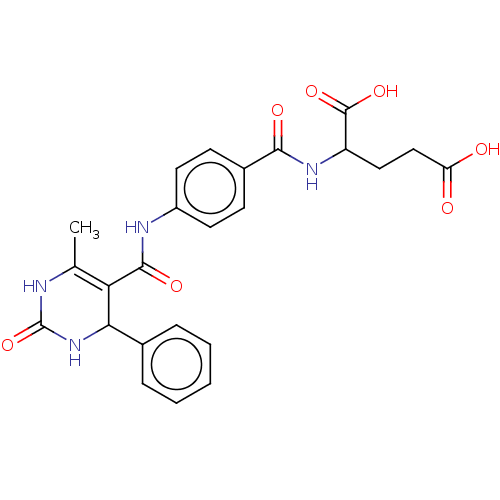
(CHEMBL4743204)Show SMILES CC1=C(C(NC(=O)N1)c1ccccc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562444

(CHEMBL4783671)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(Cc1ccccc1)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562442

(CHEMBL4748158)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(Cl)cc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562443

(CHEMBL4759800)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OCCNc1ccc(cc1)C(=O)NC(C(C)C)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562441

(CHEMBL4791810)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(cc1)[N+]([O-])=O)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562438

(CHEMBL4743204)Show SMILES CC1=C(C(NC(=O)N1)c1ccccc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562440

(CHEMBL4754677)Show SMILES COc1ccc(cc1)C1NC(=O)NC(C)=C1C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |c:15| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562449

(CHEMBL4745475)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562447

(CHEMBL4761589)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(Cc1cc2ccccc2[nH]1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50562439

(CHEMBL4750040)Show SMILES CC1=C(C(NC(=O)N1)c1ccc(C)cc1)C(=O)Nc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major DHFR in promastigote stage Leishmania major using dihydrofolic acid as substrate in presence of NADPH measured after 5... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Escherichia coli) | BDBM50179406

(CHEMBL3814850)Show SMILES CCCc1nc2ccc(Cl)cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H21ClN6/c1-2-5-23-26-21-13-12-18(25)14-22(21)31(23)15-16-8-10-17(11-9-16)19-6-3-4-7-20(19)24-27-29-30-28-24/h3-4,6-14H,2,5,15H2,1H3,(H,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Y.B. Chavan College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli peptide deformylase |
Bioorg Med Chem 24: 3456-63 (2016)
Article DOI: 10.1016/j.bmc.2016.05.051
BindingDB Entry DOI: 10.7270/Q2J67JV4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242322
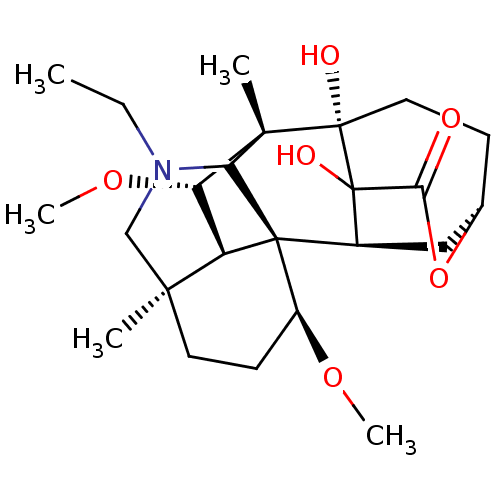
(CHEMBL4066113)Show SMILES [H][C@@]12[C@H](OC)[C@]3(C)C4N(CC)C[C@]1(C)CC[C@H](OC)[C@@]24[C@]1([H])C[C@@]2([H])CC[C@@]3(O)C1(O)C(=O)O2 |r,TLB:20:19:12.11.8:5.2,8:7:1.2:27.29.20,5:7:1:15.16.14,THB:16:19:12.11.8:5.2,31:29:19.7.5:22.23.25.26,32:31:20.22:27.25.26,30:29:19.7.5:22.23.25.26,9:8:1:15.16.14| Show InChI InChI=1S/C24H37NO6/c1-6-25-12-20(2)9-8-15(29-4)23-14-11-13-7-10-22(27,24(14,28)19(26)31-13)21(3,18(23)25)17(30-5)16(20)23/h13-18,27-28H,6-12H2,1-5H3/t13-,14-,15-,16+,17-,18?,20-,21+,22-,23-,24?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242315
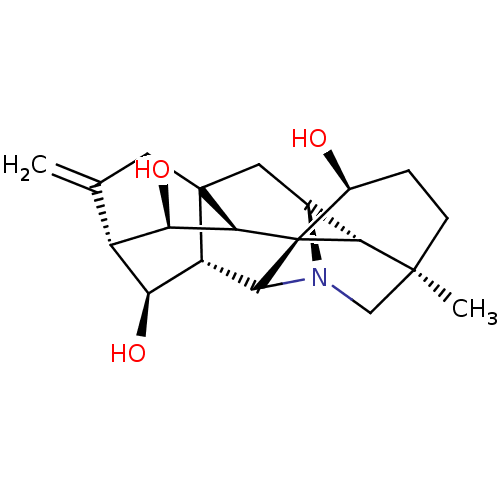
(CHEMBL4093688)Show SMILES [H][C@@]12CC34CC(=C)[C@@]5([H])[C@H](O)[C@@]3([H])C3N1C[C@]1(C)CC[C@H](O)[C@@]3([C@@]4([H])[C@@H]5O)[C@]21[H] |r,TLB:22:23:5.4:11.9,27:22:3:25.7.9,17:16:22.13:1,14:13:3:25.7.9,11:13:16.15:1,11:13:27:3.23.2,23:22:16.15:1,5:7:22.13:3,6:5:23.25:11.9,9:11:23:27.2.1,10:9:23.25:5.4,2:3:22.13:25.7.9,THB:20:22:16.15:1,20:22:3:25.7.9,18:16:22.13:1,15:14:27:3.23.2,14:13:23:27.2.1,13:11:23.25:5.4,4:3:22.13:25.7.9,10:9:22.13:3,26:25:22.13:3,26:25:5.4:11.9,2:1:16.15:22.13| Show InChI InChI=1S/C20H27NO3/c1-8-5-19-6-9-15-18(2)4-3-10(22)20(15)16(19)14(24)11(8)13(23)12(19)17(20)21(9)7-18/h9-17,22-24H,1,3-7H2,2H3/t9-,10-,11-,12-,13-,14+,15+,16-,17?,18-,19?,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242323
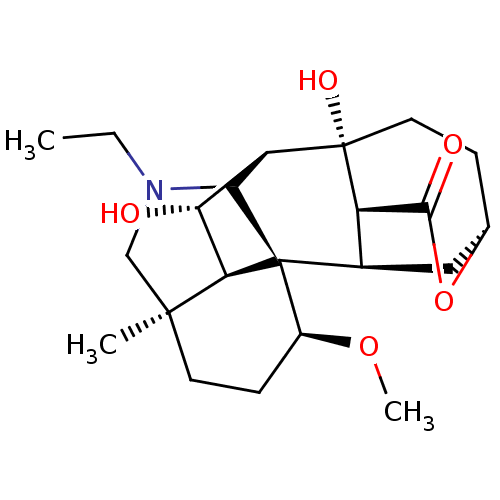
(CHEMBL4070917)Show SMILES [H][C@]12[C@@H](O)[C@@]3([H])[C@]4(C1N(CC)C[C@]3(C)CC[C@@H]4OC)[C@]1([H])C[C@@]3([H])CC[C@@]2(O)[C@@]1([H])C(=O)O3 |r,TLB:8:7:4.2:26.28.19,1:7:4:15.16.14,19:6:12.11.8:1.2,THB:16:6:12.11.8:1.2,30:28:6.7.1:21.22.24.25,31:30:19.21:26.24.25,9:8:4:15.16.14| Show InChI InChI=1S/C22H33NO5/c1-4-23-10-20(2)7-6-13(27-3)22-12-9-11-5-8-21(26,14(12)19(25)28-11)15(18(22)23)16(24)17(20)22/h11-18,24,26H,4-10H2,1-3H3/t11-,12+,13-,14+,15-,16+,17+,18?,20-,21+,22-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242317
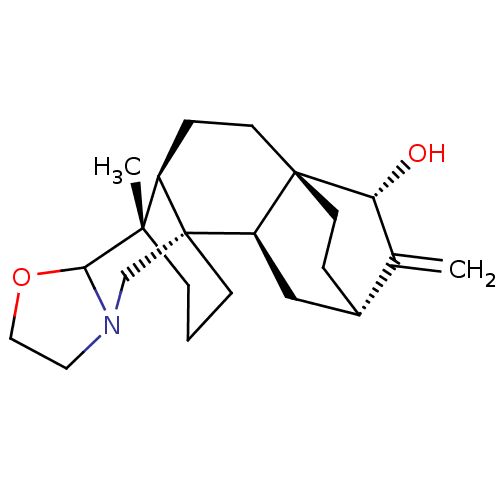
(CHEMBL4085938)Show SMILES [H][C@]12CC[C@@]3(CC[C@]4([H])[C@]5(C)CCC[C@]4(CN4CCOC54)[C@]3([H])C1)[C@]([H])(O)C2=C |r| Show InChI InChI=1S/C22H33NO2/c1-14-15-4-8-21(18(14)24)9-5-16-20(2)6-3-7-22(16,17(21)12-15)13-23-10-11-25-19(20)23/h15-19,24H,1,3-13H2,2H3/t15-,16+,17+,18+,19?,20-,21-,22+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50242322

(CHEMBL4066113)Show SMILES [H][C@@]12[C@H](OC)[C@]3(C)C4N(CC)C[C@]1(C)CC[C@H](OC)[C@@]24[C@]1([H])C[C@@]2([H])CC[C@@]3(O)C1(O)C(=O)O2 |r,TLB:20:19:12.11.8:5.2,8:7:1.2:27.29.20,5:7:1:15.16.14,THB:16:19:12.11.8:5.2,31:29:19.7.5:22.23.25.26,32:31:20.22:27.25.26,30:29:19.7.5:22.23.25.26,9:8:1:15.16.14| Show InChI InChI=1S/C24H37NO6/c1-6-25-12-20(2)9-8-15(29-4)23-14-11-13-7-10-22(27,24(14,28)19(26)31-13)21(3,18(23)25)17(30-5)16(20)23/h13-18,27-28H,6-12H2,1-5H3/t13-,14-,15-,16+,17-,18?,20-,21+,22-,23-,24?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50242315

(CHEMBL4093688)Show SMILES [H][C@@]12CC34CC(=C)[C@@]5([H])[C@H](O)[C@@]3([H])C3N1C[C@]1(C)CC[C@H](O)[C@@]3([C@@]4([H])[C@@H]5O)[C@]21[H] |r,TLB:22:23:5.4:11.9,27:22:3:25.7.9,17:16:22.13:1,14:13:3:25.7.9,11:13:16.15:1,11:13:27:3.23.2,23:22:16.15:1,5:7:22.13:3,6:5:23.25:11.9,9:11:23:27.2.1,10:9:23.25:5.4,2:3:22.13:25.7.9,THB:20:22:16.15:1,20:22:3:25.7.9,18:16:22.13:1,15:14:27:3.23.2,14:13:23:27.2.1,13:11:23.25:5.4,4:3:22.13:25.7.9,10:9:22.13:3,26:25:22.13:3,26:25:5.4:11.9,2:1:16.15:22.13| Show InChI InChI=1S/C20H27NO3/c1-8-5-19-6-9-15-18(2)4-3-10(22)20(15)16(19)14(24)11(8)13(23)12(19)17(20)21(9)7-18/h9-17,22-24H,1,3-7H2,2H3/t9-,10-,11-,12-,13-,14+,15+,16-,17?,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase alkB homolog 3
(Homo sapiens (Human)) | BDBM50463170
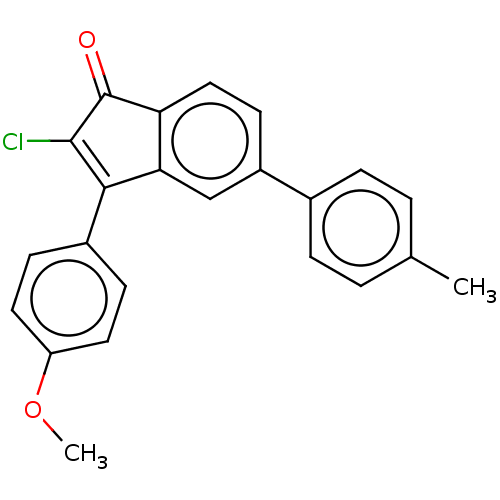
(CHEMBL4239759)Show SMILES COc1ccc(cc1)C1=C(Cl)C(=O)c2ccc(cc12)-c1ccc(C)cc1 |c:9| Show InChI InChI=1S/C23H17ClO2/c1-14-3-5-15(6-4-14)17-9-12-19-20(13-17)21(22(24)23(19)25)16-7-10-18(26-2)11-8-16/h3-13H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology Hyderabad
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AlkBH3 assessed as reduction in formaldehyde release using 40-mer single N3-meC as substrate incubated for 60 mins fo... |
Bioorg Med Chem 26: 4100-4112 (2018)
Article DOI: 10.1016/j.bmc.2018.06.040
BindingDB Entry DOI: 10.7270/Q22B91PG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242318
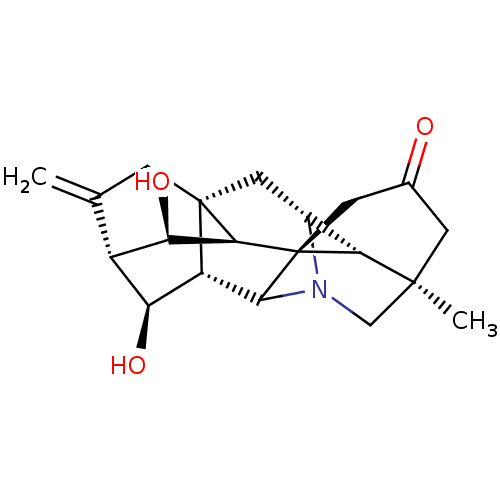
(CHEMBL4098012)Show SMILES [H][C@]12C[C@@]34CC(=C)[C@@]5([H])[C@H](O)[C@@]3([H])C3N1C[C@]1(C)CC(=O)C[C@@]3([C@@]4([H])[C@@H]5O)[C@]21[H] |r,TLB:22:23:5.4:11.9,27:22:3:25.7.9,17:16:22.13:1,14:13:3:25.7.9,11:13:16.15:1,11:13:27:3.23.2,23:22:16.15:1,25:23:13.11:27.2.1,5:7:22.13:3,6:5:23.25:11.9,10:9:23.25:5.4,2:3:22.13:25.7.9,THB:21:22:16.15:1,21:22:3:25.7.9,18:16:22.13:1,15:14:27:3.23.2,13:11:23.25:5.4,4:3:22.13:25.7.9,10:9:22.13:3,26:25:22.13:3,26:25:5.4:11.9,2:1:16.15:22.13| Show InChI InChI=1S/C20H25NO3/c1-8-3-19-6-10-15-18(2)4-9(22)5-20(15)16(19)14(24)11(8)13(23)12(19)17(20)21(10)7-18/h10-17,23-24H,1,3-7H2,2H3/t10-,11+,12+,13+,14-,15-,16+,17?,18+,19+,20+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50242324

(CHEMBL4072441)Show SMILES [H][C@]12OCCN1C[C@]13CCC[C@@]2(C)[C@@]1([H])CC[C@@]12CC[C@@]([H])(C[C@@]31[H])C(=C)[C@@]2([H])O |r| Show InChI InChI=1S/C22H33NO2/c1-14-15-4-8-21(18(14)24)9-5-16-20(2)6-3-7-22(16,17(21)12-15)13-23-10-11-25-19(20)23/h15-19,24H,1,3-13H2,2H3/t15-,16+,17+,18+,19+,20-,21-,22+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50242323

(CHEMBL4070917)Show SMILES [H][C@]12[C@@H](O)[C@@]3([H])[C@]4(C1N(CC)C[C@]3(C)CC[C@@H]4OC)[C@]1([H])C[C@@]3([H])CC[C@@]2(O)[C@@]1([H])C(=O)O3 |r,TLB:8:7:4.2:26.28.19,1:7:4:15.16.14,19:6:12.11.8:1.2,THB:16:6:12.11.8:1.2,30:28:6.7.1:21.22.24.25,31:30:19.21:26.24.25,9:8:4:15.16.14| Show InChI InChI=1S/C22H33NO5/c1-4-23-10-20(2)7-6-13(27-3)22-12-9-11-5-8-21(26,14(12)19(25)28-11)15(18(22)23)16(24)17(20)22/h11-18,24,26H,4-10H2,1-3H3/t11-,12+,13-,14+,15-,16+,17+,18?,20-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50562446

(CHEMBL4752301)Show SMILES COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC(=O)CNC(CC(C)C)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human DHFR measured by spectrophotometric method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112986
BindingDB Entry DOI: 10.7270/Q2SF30W1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... |
Bioorg Med Chem 25: 3368-3376 (2017)
Article DOI: 10.1016/j.bmc.2017.04.022
BindingDB Entry DOI: 10.7270/Q2K64MG6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data