 Found 45 hits with Last Name = 'chang' and Initial = 'g'
Found 45 hits with Last Name = 'chang' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50272772

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES C[C@@H](O)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kuang Tien General Hospital
Curated by ChEMBL
| Assay Description
Binding affinity to human SSTR2A |
Bioorg Med Chem Lett 18: 4593-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.027
BindingDB Entry DOI: 10.7270/Q2SQ9063 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023636
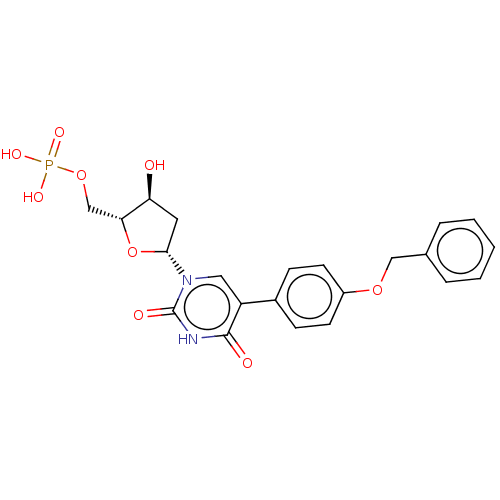
(CHEMBL3144190 | Phosphoric acid mono-{5-[5-(4-benz...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(OCc3ccccc3)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H23N2O9P/c25-18-10-20(33-19(18)13-32-34(28,29)30)24-11-17(21(26)23-22(24)27)15-6-8-16(9-7-15)31-12-14-4-2-1-3-5-14/h1-9,11,18-20,25H,10,12-13H2,(H,23,26,27)(H2,28,29,30)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023645
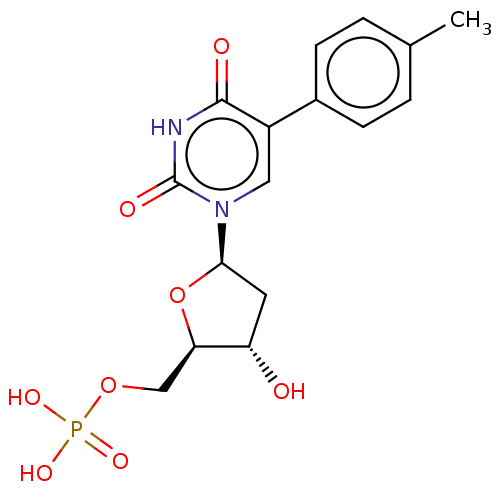
(CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES Cc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O8P/c1-9-2-4-10(5-3-9)11-7-18(16(21)17-15(11)20)14-6-12(19)13(26-14)8-25-27(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023647
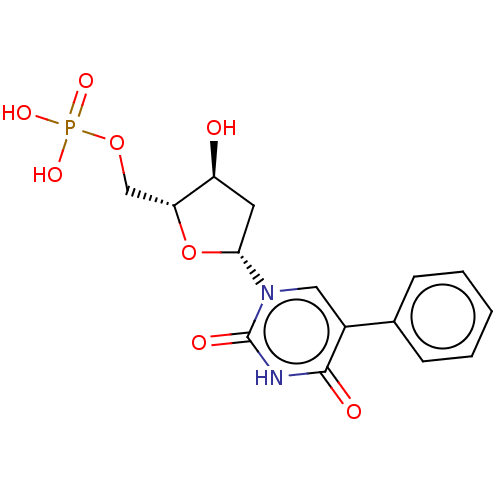
(CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccccc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O8P/c18-11-6-13(25-12(11)8-24-26(21,22)23)17-7-10(14(19)16-15(17)20)9-4-2-1-3-5-9/h1-5,7,11-13,18H,6,8H2,(H,16,19,20)(H2,21,22,23)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023637

(CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...)Show SMILES Nc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H18N3O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7,16H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023640
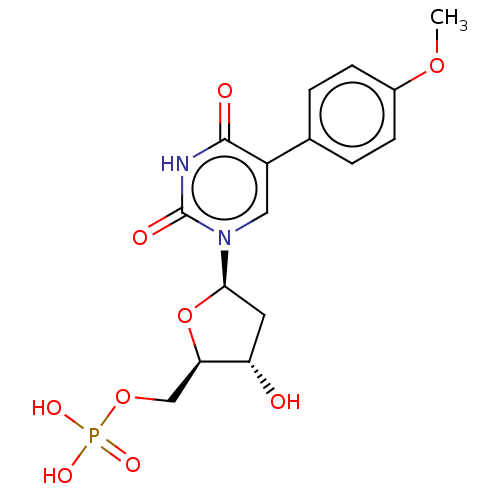
(CHEMBL3144188 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES COc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O9P/c1-25-10-4-2-9(3-5-10)11-7-18(16(21)17-15(11)20)14-6-12(19)13(27-14)8-26-28(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023639
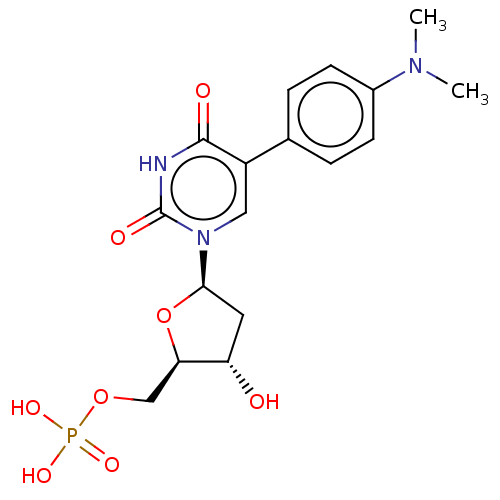
(CHEMBL3144192 | Phosphoric acid mono-{5-[5-(4-dime...)Show SMILES CN(C)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H22N3O8P/c1-19(2)11-5-3-10(4-6-11)12-8-20(17(23)18-16(12)22)15-7-13(21)14(28-15)9-27-29(24,25)26/h3-6,8,13-15,21H,7,9H2,1-2H3,(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023641

(CHEMBL3144196 | Phosphoric acid mono-{5-[5-(4-brom...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(Br)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16BrN2O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023635

(4-[1-(4-Hydroxy-5-phosphonooxymethyl-tetrahydro-fu...)Show SMILES COC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H19N2O10P/c1-27-16(22)10-4-2-9(3-5-10)11-7-19(17(23)18-15(11)21)14-6-12(20)13(29-14)8-28-30(24,25)26/h2-5,7,12-14,20H,6,8H2,1H3,(H,18,21,23)(H2,24,25,26)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023638
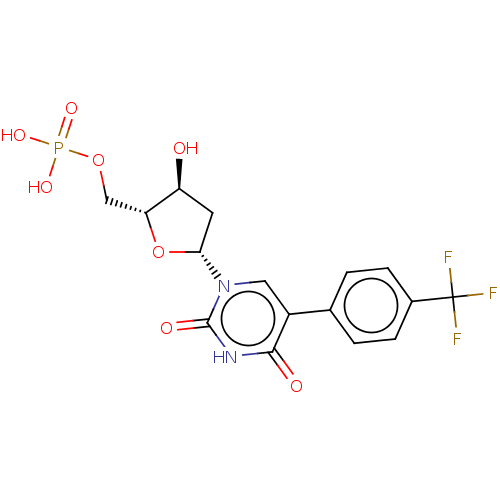
(CHEMBL3144194 | Phosphoric acid mono-{5-[2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C(F)(F)F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16F3N2O8P/c17-16(18,19)9-3-1-8(2-4-9)10-6-21(15(24)20-14(10)23)13-5-11(22)12(29-13)7-28-30(25,26)27/h1-4,6,11-13,22H,5,7H2,(H,20,23,24)(H2,25,26,27)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023645

(CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES Cc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O8P/c1-9-2-4-10(5-3-9)11-7-18(16(21)17-15(11)20)14-6-12(19)13(26-14)8-25-27(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023643
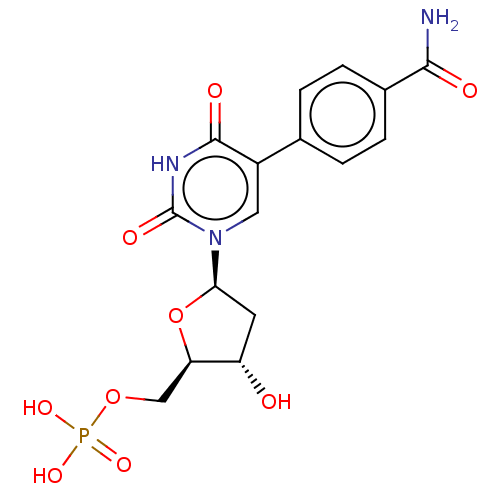
(CHEMBL3144195 | Phosphoric acid mono-{5-[5-(4-carb...)Show SMILES NC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H18N3O9P/c17-14(21)9-3-1-8(2-4-9)10-6-19(16(23)18-15(10)22)13-5-11(20)12(28-13)7-27-29(24,25)26/h1-4,6,11-13,20H,5,7H2,(H2,17,21)(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50227388
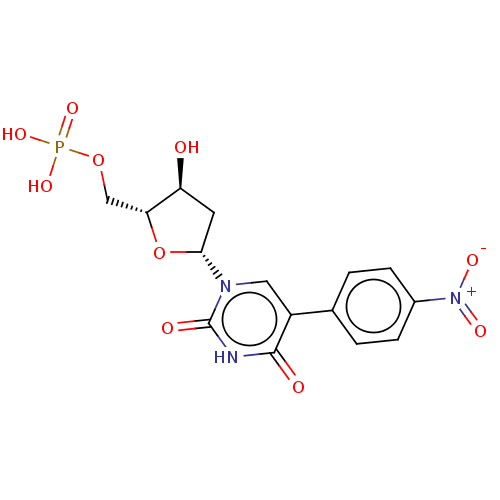
(CHEMBL3144092)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)[N+]([O-])=O)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16N3O10P/c19-11-5-13(28-12(11)7-27-29(24,25)26)17-6-10(14(20)16-15(17)21)8-1-3-9(4-2-8)18(22)23/h1-4,6,11-13,19H,5,7H2,(H,16,20,21)(H2,24,25,26)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023647

(CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccccc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O8P/c18-11-6-13(25-12(11)8-24-26(21,22)23)17-7-10(14(19)16-15(17)20)9-4-2-1-3-5-9/h1-5,7,11-13,18H,6,8H2,(H,16,19,20)(H2,21,22,23)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023644
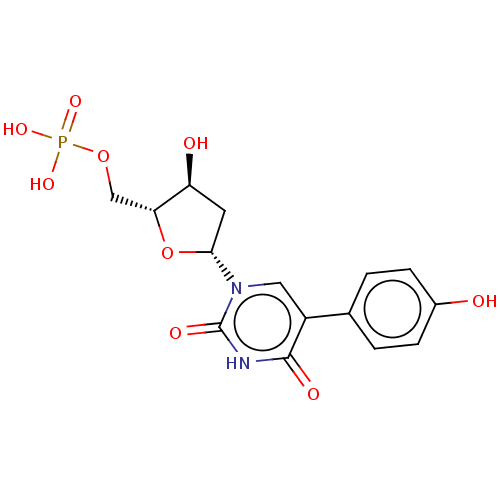
(CHEMBL3144191 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(O)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O9P/c18-9-3-1-8(2-4-9)10-6-17(15(21)16-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,18-19H,5,7H2,(H,16,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Mus musculus) | BDBM50023645

(CHEMBL3144187 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES Cc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O8P/c1-9-2-4-10(5-3-9)11-7-18(16(21)17-15(11)20)14-6-12(19)13(26-14)8-25-27(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50023642
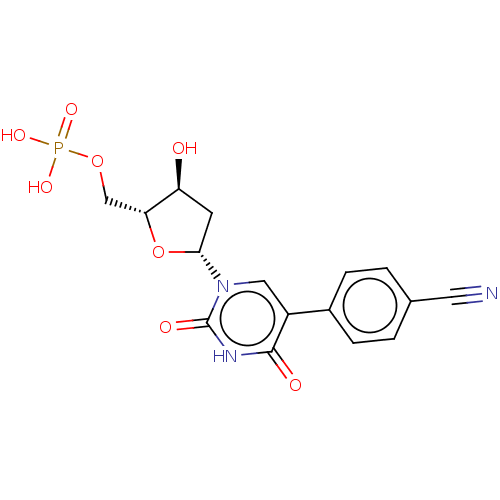
(CHEMBL3144193 | Phosphoric acid mono-{5-[5-(4-cyan...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C#N)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16N3O8P/c17-6-9-1-3-10(4-2-9)11-7-19(16(22)18-15(11)21)14-5-12(20)13(27-14)8-26-28(23,24)25/h1-4,7,12-14,20H,5,8H2,(H,18,21,22)(H2,23,24,25)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on Lactobacillus casei thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Mus musculus) | BDBM50023636

(CHEMBL3144190 | Phosphoric acid mono-{5-[5-(4-benz...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(OCc3ccccc3)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H23N2O9P/c25-18-10-20(33-19(18)13-32-34(28,29)30)24-11-17(21(26)23-22(24)27)15-6-8-16(9-7-15)31-12-14-4-2-1-3-5-14/h1-9,11,18-20,25H,10,12-13H2,(H,23,26,27)(H2,28,29,30)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023637

(CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...)Show SMILES Nc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H18N3O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7,16H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023647

(CHEMBL3144186 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccccc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O8P/c18-11-6-13(25-12(11)8-24-26(21,22)23)17-7-10(14(19)16-15(17)20)9-4-2-1-3-5-9/h1-5,7,11-13,18H,6,8H2,(H,16,19,20)(H2,21,22,23)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023637

(CHEMBL3144189 | Phosphoric acid mono-{5-[5-(4-amin...)Show SMILES Nc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H18N3O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7,16H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023641

(CHEMBL3144196 | Phosphoric acid mono-{5-[5-(4-brom...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(Br)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16BrN2O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023641

(CHEMBL3144196 | Phosphoric acid mono-{5-[5-(4-brom...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(Br)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16BrN2O8P/c16-9-3-1-8(2-4-9)10-6-18(15(21)17-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,19H,5,7H2,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023636

(CHEMBL3144190 | Phosphoric acid mono-{5-[5-(4-benz...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(OCc3ccccc3)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C22H23N2O9P/c25-18-10-20(33-19(18)13-32-34(28,29)30)24-11-17(21(26)23-22(24)27)15-6-8-16(9-7-15)31-12-14-4-2-1-3-5-14/h1-9,11,18-20,25H,10,12-13H2,(H,23,26,27)(H2,28,29,30)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023640

(CHEMBL3144188 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES COc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O9P/c1-25-10-4-2-9(3-5-10)11-7-18(16(21)17-15(11)20)14-6-12(19)13(27-14)8-26-28(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023638

(CHEMBL3144194 | Phosphoric acid mono-{5-[2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C(F)(F)F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16F3N2O8P/c17-16(18,19)9-3-1-8(2-4-9)10-6-21(15(24)20-14(10)23)13-5-11(22)12(29-13)7-28-30(25,26)27/h1-4,6,11-13,22H,5,7H2,(H,20,23,24)(H2,25,26,27)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023640

(CHEMBL3144188 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES COc1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H19N2O9P/c1-25-10-4-2-9(3-5-10)11-7-18(16(21)17-15(11)20)14-6-12(19)13(27-14)8-26-28(22,23)24/h2-5,7,12-14,19H,6,8H2,1H3,(H,17,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023635

(4-[1-(4-Hydroxy-5-phosphonooxymethyl-tetrahydro-fu...)Show SMILES COC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H19N2O10P/c1-27-16(22)10-4-2-9(3-5-10)11-7-19(17(23)18-15(11)21)14-6-12(20)13(29-14)8-28-30(24,25)26/h2-5,7,12-14,20H,6,8H2,1H3,(H,18,21,23)(H2,24,25,26)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023639

(CHEMBL3144192 | Phosphoric acid mono-{5-[5-(4-dime...)Show SMILES CN(C)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H22N3O8P/c1-19(2)11-5-3-10(4-6-11)12-8-20(17(23)18-16(12)22)15-7-13(21)14(28-15)9-27-29(24,25)26/h3-6,8,13-15,21H,7,9H2,1-2H3,(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50227388

(CHEMBL3144092)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)[N+]([O-])=O)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16N3O10P/c19-11-5-13(28-12(11)7-27-29(24,25)26)17-6-10(14(20)16-15(17)21)8-1-3-9(4-2-8)18(22)23/h1-4,6,11-13,19H,5,7H2,(H,16,20,21)(H2,24,25,26)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50227388

(CHEMBL3144092)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)[N+]([O-])=O)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H16N3O10P/c19-11-5-13(28-12(11)7-27-29(24,25)26)17-6-10(14(20)16-15(17)21)8-1-3-9(4-2-8)18(22)23/h1-4,6,11-13,19H,5,7H2,(H,16,20,21)(H2,24,25,26)/t11-,12+,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023639

(CHEMBL3144192 | Phosphoric acid mono-{5-[5-(4-dime...)Show SMILES CN(C)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H22N3O8P/c1-19(2)11-5-3-10(4-6-11)12-8-20(17(23)18-16(12)22)15-7-13(21)14(28-15)9-27-29(24,25)26/h3-6,8,13-15,21H,7,9H2,1-2H3,(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023638

(CHEMBL3144194 | Phosphoric acid mono-{5-[2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C(F)(F)F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16F3N2O8P/c17-16(18,19)9-3-1-8(2-4-9)10-6-21(15(24)20-14(10)23)13-5-11(22)12(29-13)7-28-30(25,26)27/h1-4,6,11-13,22H,5,7H2,(H,20,23,24)(H2,25,26,27)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023642

(CHEMBL3144193 | Phosphoric acid mono-{5-[5-(4-cyan...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C#N)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16N3O8P/c17-6-9-1-3-10(4-2-9)11-7-19(16(22)18-15(11)21)14-5-12(20)13(27-14)8-26-28(23,24)25/h1-4,7,12-14,20H,5,8H2,(H,18,21,22)(H2,23,24,25)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023635

(4-[1-(4-Hydroxy-5-phosphonooxymethyl-tetrahydro-fu...)Show SMILES COC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H19N2O10P/c1-27-16(22)10-4-2-9(3-5-10)11-7-19(17(23)18-15(11)21)14-6-12(20)13(29-14)8-28-30(24,25)26/h2-5,7,12-14,20H,6,8H2,1H3,(H,18,21,23)(H2,24,25,26)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50023643

(CHEMBL3144195 | Phosphoric acid mono-{5-[5-(4-carb...)Show SMILES NC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H18N3O9P/c17-14(21)9-3-1-8(2-4-9)10-6-19(16(23)18-15(10)22)13-5-11(20)12(28-13)7-27-29(24,25)26/h1-4,6,11-13,20H,5,7H2,(H2,17,21)(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023642

(CHEMBL3144193 | Phosphoric acid mono-{5-[5-(4-cyan...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(cc2)C#N)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H16N3O8P/c17-6-9-1-3-10(4-2-9)11-7-19(16(22)18-15(11)21)14-5-12(20)13(27-14)8-26-28(23,24)25/h1-4,7,12-14,20H,5,8H2,(H,18,21,22)(H2,23,24,25)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023643

(CHEMBL3144195 | Phosphoric acid mono-{5-[5-(4-carb...)Show SMILES NC(=O)c1ccc(cc1)-c1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C16H18N3O9P/c17-14(21)9-3-1-8(2-4-9)10-6-19(16(23)18-15(10)22)13-5-11(20)12(28-13)7-27-29(24,25)26/h1-4,6,11-13,20H,5,7H2,(H2,17,21)(H,18,22,23)(H2,24,25,26)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023644

(CHEMBL3144191 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(O)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O9P/c18-9-3-1-8(2-4-9)10-6-17(15(21)16-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,18-19H,5,7H2,(H,16,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Mus musculus) | BDBM50023644

(CHEMBL3144191 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(-c2ccc(O)cc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H17N2O9P/c18-9-3-1-8(2-4-9)10-6-17(15(21)16-14(10)20)13-5-11(19)12(26-13)7-25-27(22,23)24/h1-4,6,11-13,18-19H,5,7H2,(H,16,20,21)(H2,22,23,24)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory effect on murine leukemia (L1210) thymidylate synthase |
J Med Chem 31: 1141-7 (1988)
BindingDB Entry DOI: 10.7270/Q2ZS2X3H |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50041289
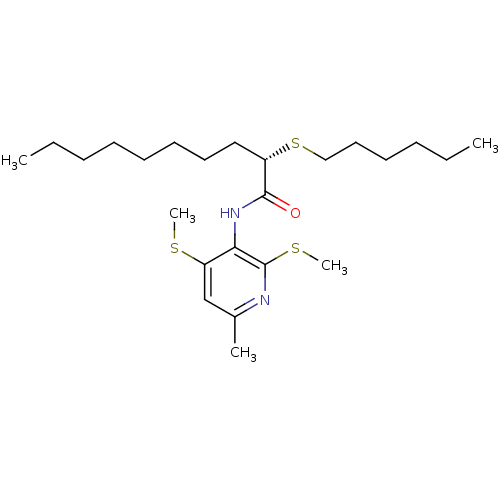
((S)-2-Hexylsulfanyl-decanoic acid (6-methyl-2,4-bi...)Show SMILES CCCCCCCC[C@H](SCCCCCC)C(=O)Nc1c(SC)cc(C)nc1SC Show InChI InChI=1S/C24H42N2OS3/c1-6-8-10-12-13-14-16-20(30-17-15-11-9-7-2)23(27)26-22-21(28-4)18-19(3)25-24(22)29-5/h18,20H,6-17H2,1-5H3,(H,26,27)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats |
J Med Chem 37: 1252-5 (1994)
BindingDB Entry DOI: 10.7270/Q25X280J |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50041290
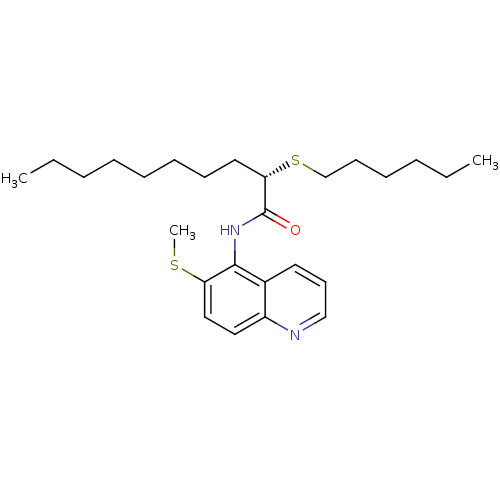
((S)-2-Hexylsulfanyl-decanoic acid (6-methylsulfany...)Show SMILES CCCCCCCC[C@H](SCCCCCC)C(=O)Nc1c(SC)ccc2ncccc12 Show InChI InChI=1S/C26H40N2OS2/c1-4-6-8-10-11-12-16-24(31-20-13-9-7-5-2)26(29)28-25-21-15-14-19-27-22(21)17-18-23(25)30-3/h14-15,17-19,24H,4-13,16,20H2,1-3H3,(H,28,29)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats |
J Med Chem 37: 1252-5 (1994)
BindingDB Entry DOI: 10.7270/Q25X280J |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-1
(Canis familiaris) | BDBM50605867
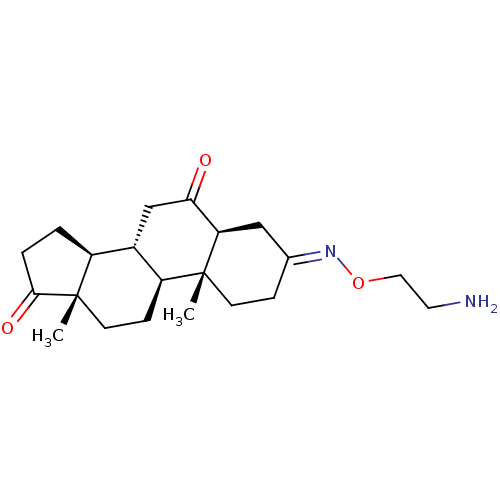
(CHEMBL469045)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC(=O)[C@@]2([H])C\C(CC[C@]12C)=N\OCCN |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00347
BindingDB Entry DOI: 10.7270/Q2JM2FQB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50453642
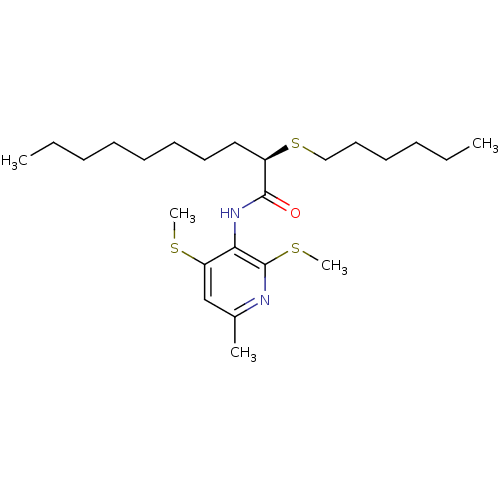
(CHEMBL2093028)Show SMILES CCCCCCCC[C@@H](SCCCCCC)C(=O)Nc1c(SC)cc(C)nc1SC Show InChI InChI=1S/C24H42N2OS3/c1-6-8-10-12-13-14-16-20(30-17-15-11-9-7-2)23(27)26-22-21(28-4)18-19(3)25-24(22)29-5/h18,20H,6-17H2,1-5H3,(H,26,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats |
J Med Chem 37: 1252-5 (1994)
BindingDB Entry DOI: 10.7270/Q25X280J |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Rattus norvegicus) | BDBM50453641
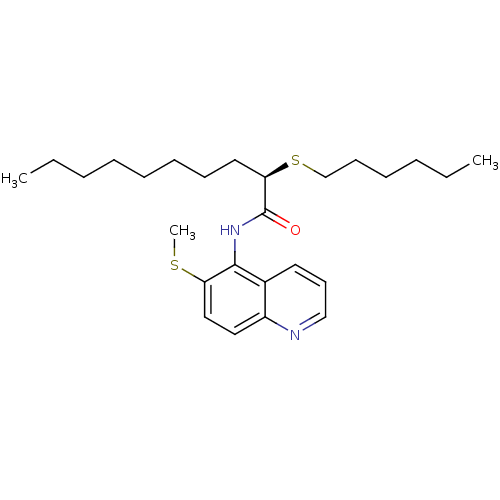
(CHEMBL2093029)Show SMILES CCCCCCCC[C@@H](SCCCCCC)C(=O)Nc1c(SC)ccc2ncccc12 Show InChI InChI=1S/C26H40N2OS2/c1-4-6-8-10-11-12-16-24(31-20-13-9-7-5-2)26(29)28-25-21-15-14-19-27-22(21)17-18-23(25)30-3/h14-15,17-19,24H,4-13,16,20H2,1-3H3,(H,28,29)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro potency was determined using Acyl coenzyme A:cholesterol acyltransferase in liver microsomes from rats |
J Med Chem 37: 1252-5 (1994)
BindingDB Entry DOI: 10.7270/Q25X280J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data