

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458625 (CHEMBL4218013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458621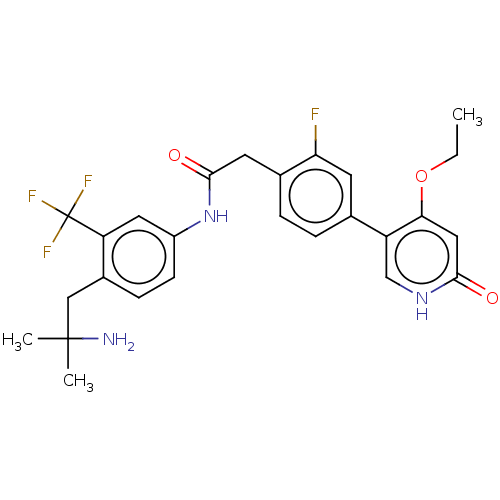 (CHEMBL4218563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458627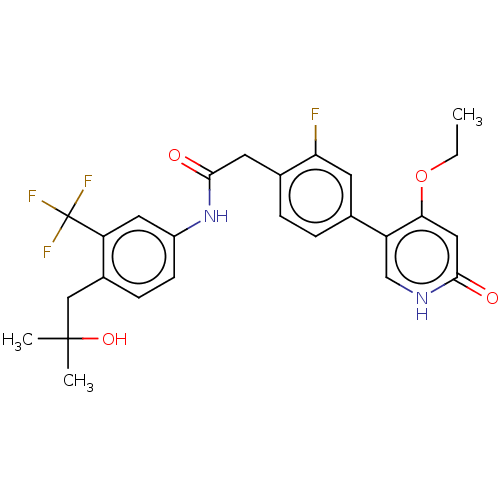 (CHEMBL4218127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347342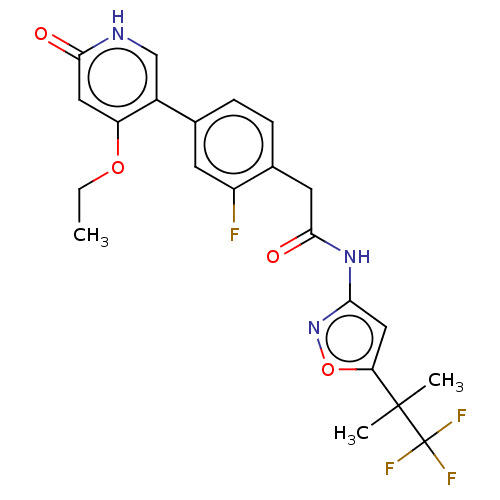 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347313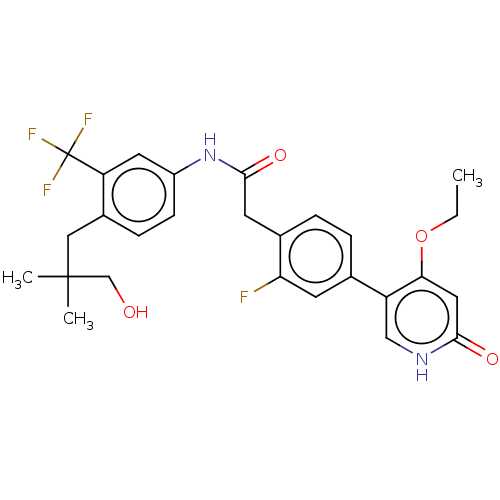 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458628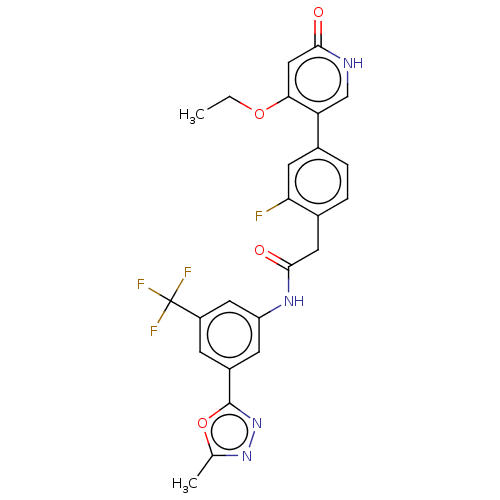 (CHEMBL4212513) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347339 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458630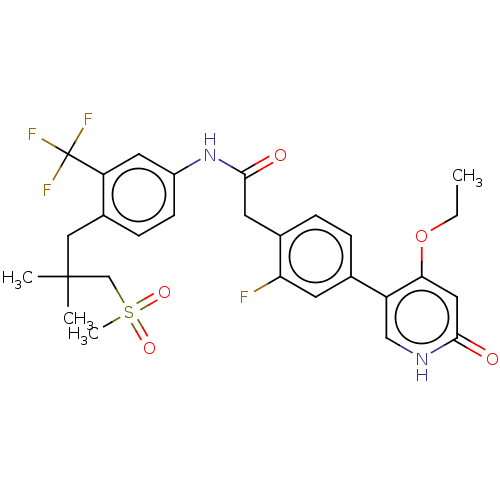 (CHEMBL4203429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458626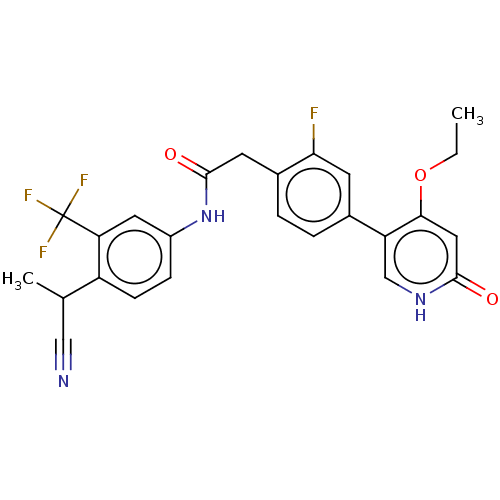 (CHEMBL4214146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458624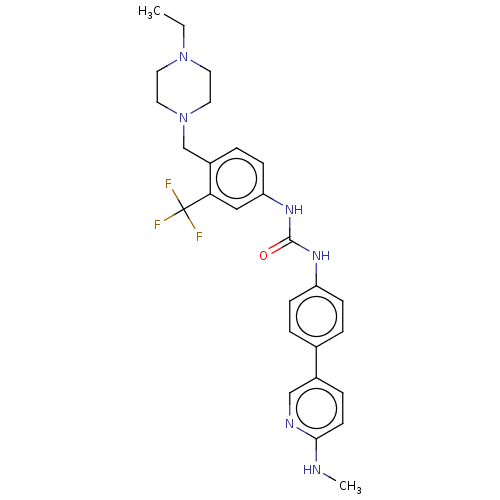 (CHEMBL4205803) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458622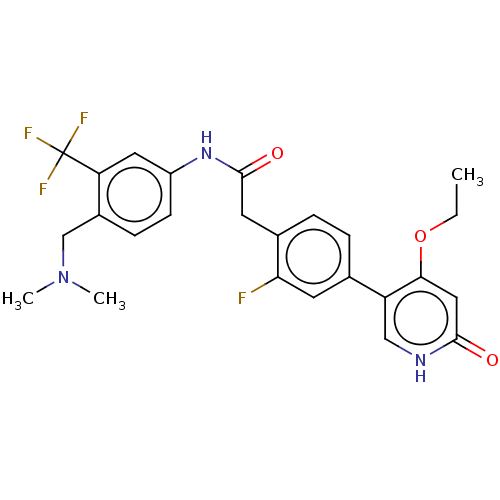 (CHEMBL4213626) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458629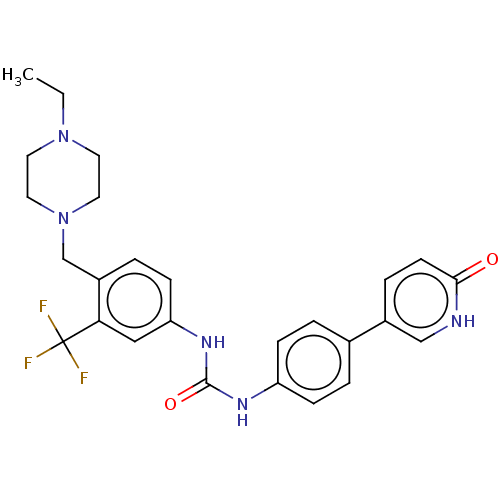 (CHEMBL4210670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347345 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458631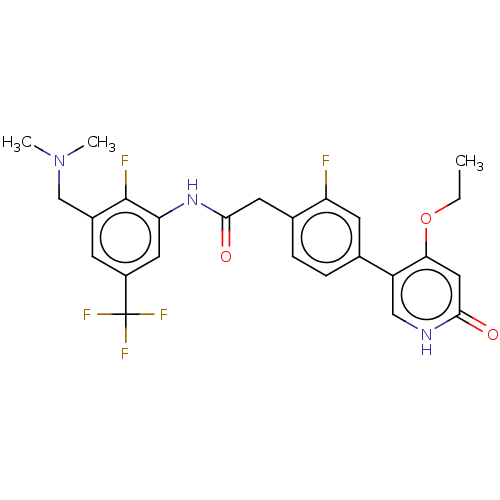 (CHEMBL4209229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132G] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347339 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458630 (CHEMBL4203429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458624 (CHEMBL4205803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458621 (CHEMBL4218563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458627 (CHEMBL4218127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458621 (CHEMBL4218563) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458627 (CHEMBL4218127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347342 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458622 (CHEMBL4213626) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458625 (CHEMBL4218013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458630 (CHEMBL4203429) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458623 (CHEMBL4215099) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458628 (CHEMBL4212513) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4E (Homo sapiens (Human)) | BDBM50153318 (CHEMBL3775237) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4E (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4E (Homo sapiens (Human)) | BDBM50153322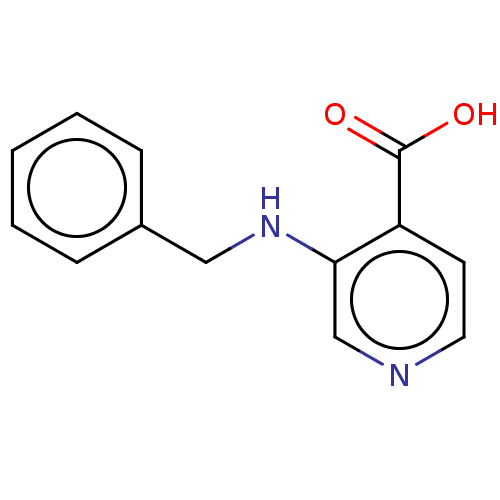 (CHEMBL3775612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4E (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM347339 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description For WT IDH1 and IDH2, reactions were conducted at room temperature in 384-well Greiner black microtiter plates in a total volume of 10 μL of ass... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4E (Homo sapiens (Human)) | BDBM50153319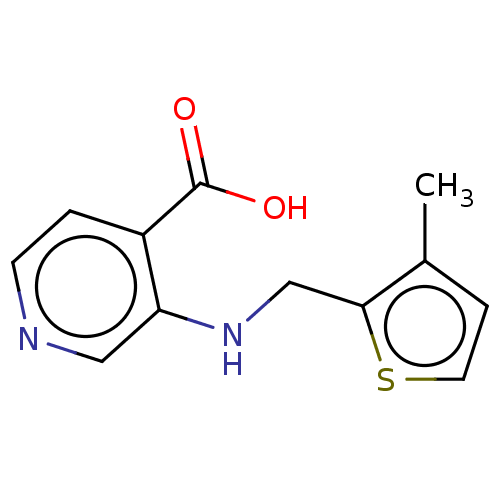 (CHEMBL3775889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4E (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM195612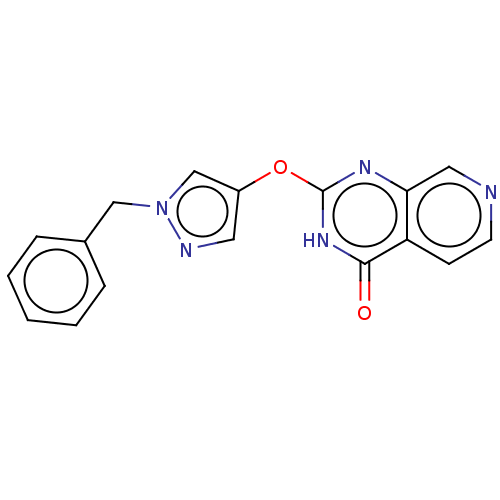 (GSK467) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM5C catalytic domain (1 -764 aa) (unknown origin) expressed in Sf9 cells using H3K4Me3 peptide as substrate by RFMS assay | J Med Chem 59: 1370-87 (2016) Article DOI: 10.1021/acs.jmedchem.5b01538 BindingDB Entry DOI: 10.7270/Q26D5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4D (Homo sapiens (Human)) | BDBM50153322 (CHEMBL3775612) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human KDM4D (11 to 341 residues) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectromet... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153331 (CHEMBL3774552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153330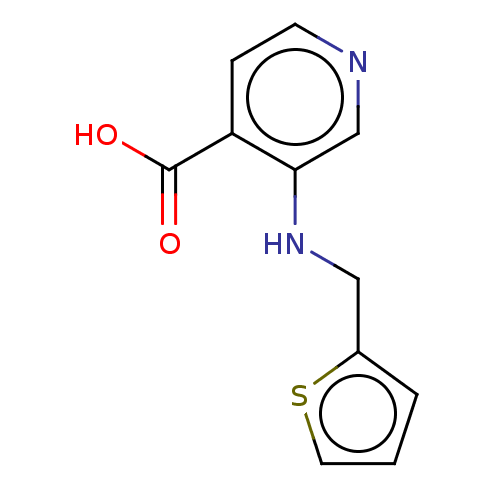 (CHEMBL3774859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458626 (CHEMBL4214146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM347345 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458631 (CHEMBL4209229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153319 (CHEMBL3775889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50149917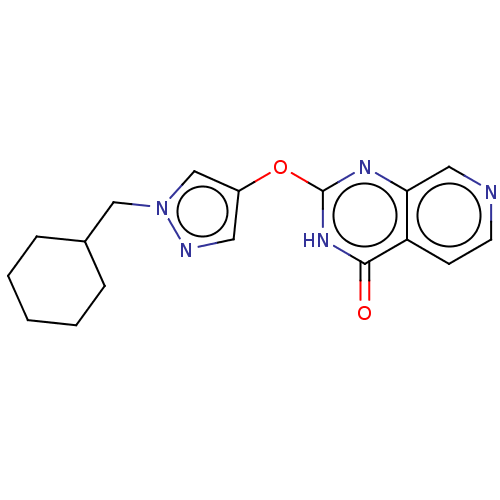 (CHEMBL3771198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM5C catalytic domain (1 -764 aa) (unknown origin) expressed in Sf9 cells using H3K4Me3 peptide as substrate by RFMS assay | J Med Chem 59: 1370-87 (2016) Article DOI: 10.1021/acs.jmedchem.5b01538 BindingDB Entry DOI: 10.7270/Q26D5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153318 (CHEMBL3775237) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458626 (CHEMBL4214146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50458629 (CHEMBL4210670) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50153332 (CHEMBL3775439) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of KDM4C (unknown origin) using H3K9Me3 peptide as substrate assessed as demethylation of substrate by Rapidfire mass spectrometric analys... | J Med Chem 59: 1357-69 (2016) Article DOI: 10.1021/acs.jmedchem.5b01537 BindingDB Entry DOI: 10.7270/Q2542QFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 286 total ) | Next | Last >> |